Plasmolifting (PRP, Plateletrichplasma) is an injection method of subcutaneous administration of platelet-rich plasma from the patient’s own blood, leading to the activation of regenerative processes in the skin. Human blood plasma contains the substances fibrin and collagen. They are the ones who participate in the construction of new tissue cells in the body. These substances help restore cells of any organ, be it skin that has lost its elasticity or brittle hair. The essence of the method is to isolate plasma from human blood and then inject it into the area requiring restoration.
In addition, plasma has a high concentration of platelets, which contain special growth factors and proteins. They stimulate the production of fibroblasts in stem cells, which enhances the regeneration process.
Mechanism of action of plasma lifting
To use this method of cell regeneration, platelet autoplasma (TAP) is required. It does not contain red blood cells, and the number of platelets in it is close to their number in peripheral blood (150-350 thousand/μl). Such plasma is very rich in amino acids, mineral elements (potassium, magnesium, zinc, iron, calcium, etc., peptides and vitamins B, C, A, D, E, K, necessary for the life of cells. Being a natural component of the human body , autoplasma does not cause allergic reactions. This method is also universal due to its bioavailability, due to which regeneration processes occur naturally.
Physiologically, the process of plasmolifting occurs as follows: when platelets lose contact with the endothelium, leaving the capillaries and vessels, they change their shape and secrete alpha granules, which saturate the damaged area with growth factors.
The same thing happens in the plasmolifting procedure, when autoplasma is injected into the desired area, i.e. platelet adhesion and the release of growth factors from alpha granules occur, as in a normal protective physiological process.
The essence of the method
Autohemotherapy is a therapeutic technique that involves injecting the patient with his own blood. In most cases it is performed intramuscularly.
In essence, this is a transfusion that enhances hematopoiesis and increases the body's resistance to all kinds of diseases.
Scientists believe that human blood circulating through vessels is able to “remember” the processes occurring in the body. After the secondary entry into the body, the cells independently identify sources of inflammation and pathogenic microorganisms, destroying them.
Based on existing reviews from doctors, indications and contraindications, we can conclude that autohemotherapy is aimed at:
- increasing the body's immune defense;
- activation of hematopoietic processes and the hemostatic system.
After using the technique, there are usually no chills, joint damage, increased body temperature and other negative manifestations. The use of autohemotherapy is relevant not only for inpatient settings, but also for outpatient settings.
How does the plasma lifting procedure work?
Blood taken from the patient is subjected to a centrifugation procedure to separate red blood cells. Platelet autoplasma should be yellow. After 10-15 minutes, the prepared platelet autoplasma can already be used as an injection into the area required for treatment. Before this, the skin is treated with a special anesthetic cream. A thin needle is taken and the drug is injected using the papular method.
The course of treatment using plasma lifting is prescribed individually, based on the initial condition and degree of cell damage. On average, 3-4-5 procedures are recommended with an interval of about a week - such a course produces a noticeable effect. To maintain improved cell condition, you can repeat the procedure once every year or two.
Reviews
JULIA, 53 YEARS OLD:
“For several years I suffered from severe symptoms of menopause.
My blood pressure was skyrocketing, I was often dizzy, I didn’t sleep well and constantly felt tired. The doctor advised me to undergo autohemotherapy and talked about the indications and contraindications of the technique in gynecology. At first it was a little scary, but then I decided to try everything.
After the course of injections, I noticed positive changes: more energy appeared, dizziness went away, and my ability to work increased. I recommend!"
ANNA, 24 YEARS OLD:
“My main problem since adolescence has been acne, and what I haven’t done about it.
Still, ugly ulcers appeared on the face, and then they spread to the back and arms. It became completely impossible to tolerate this, and I went to a dermatologist, who prescribed autohemotherapy.
They do it for free at the clinic, and already halfway through the course I noticed an improvement in my condition. There are no side effects, the results can’t help but rejoice.”
Indications for plasma lifting
In cosmetology, the use of plasma lifting is advisable in the following cases:
- loss of skin elasticity, deterioration of its turgor;
- unwanted skin pigmentation;
- noticeable facial wrinkles, the appearance of age-related folds on the skin;
- sagging face, deterioration of facial oval;
- gray, “tired” complexion;
- post-acne and acne;
- scars and stretch marks on the skin;
- hair loss (alopecia) and deterioration of their condition.
Of course, the use of autoplasma in cosmetology is not limited only to the face and scalp; these reasons are only the main ones. The method is used on any part of the human body that has defects. Body plasma lifting is a popular procedure. It is used locally to treat cellulite and obesity and rejuvenate the skin of the body.
Contraindications for plasma lifting
Despite the maximum naturalness of this method and the greatest proximity of autoplasma to the components of the blood of a certain person, the cosmetic procedure of plasma lifting has a certain number of contraindications. Among them are:
- Purulent-infectious skin diseases;
- Diabetes;
- Bleeding disorders and blood diseases;
- Decreased immunity;
- Oncology;
- Hepatitis;
- Diseases of the kidneys and pancreas.
At the same time, plasma lifting has a minimal risk of complications and side effects, because with this method no foreign substances are introduced into the body. This is its main difference from biorevitalization and mesotherapy procedures.
The individual effect of using this method is due to the dependence on the initial state of the cells. Plasmolifting is attractive primarily for its simplicity and naturalness, as well as the minimal risk of side effects. The skin is not injured. What is better biorevitalization. mesotherapy or plasma lifting for each specific patient can only be determined by a cosmetologist.
What is autohemotherapy
The procedure is based on transfusion of the patient's own blood. The material is taken from a vein and injected intramuscularly, less often – subcutaneously. The method is not new: it began to be used in the 2000s, that is, more than a century ago. During the war period, when there were no antibiotics for inflammation from wounds or the consequences of surgical operations, complex colds were practically impossible to cure. People were dying. One of the “pioneers” of autohemotherapy, Voino-Yasenetsky, in his “Essays on Purulent Surgery” of 1900, notes the effectiveness of the treatment of inflammatory and indolent diseases.
The effect of plasma lifting
Most patients notice a noticeable positive effect after 1-1.5 weeks. Some note that the effectiveness of plasma lifting occurs after the first injection. The result depends on age, skin condition and characteristics of the body as a whole.
Among those who have tried the plasmolifting method on themselves, the majority leave good reviews about the procedure, which will help shape the attitude towards this method in new patients. Many women note that the result of the procedure is skin rejuvenation, a fresher appearance, gradual smoothing of scars and a reduction in the effects of acne. The skin becomes more elastic and tightened.
Some patients complain of swelling and bruising on the skin after using an autoplasma injection, but in fact this is an obvious consequence of the cosmetic procedure. Side effects from plasma lifting are extremely rare and are usually associated with a violation of the procedure protocol or a violation of the rules of asepsis and antisepsis. Some are confused by the fact that for a noticeable effect, not one, but a certain number of injections are required, and the cost of such a procedure may not be affordable for everyone.
The plasmolifting technique has: A rejuvenating effect.
Helps remove dead cells, promoting the formation of new ones, smoothes the skin, increasing its turgor, the skin becomes tender and soft to the touch. Activates the BCL-2 gene, which delays cell aging.
Protective effect.
Strengthens the protective barrier against the effects of harmful ultraviolet radiation, improves skin regeneration, due to which small wounds and scars heal faster. The skin becomes protected from the effects of all kinds of external factors that have a negative impact on its condition: temperature changes, sunlight, the consequences of taking strong medications. Suppresses the proliferation of pathogenic microflora.
Moisturizing effect.
Prevents skin dehydration. The skin is deeply hydrated and the synthesis of macromolecular proteins, which provide skin elasticity, is improved. Skin cells retain more moisture inside, which eliminates the feeling of tightness, dryness, and reduces the appearance of skin irritation and inflammation.
Reduces skin photosensitivity.
The appearance improves due to increased cell metabolism, which prevents the appearance of age spots after sun exposure, which leads to an even complexion.
Improves skin trophism.
The process of blood circulation in the dermis is enhanced, metabolic processes are accelerated, which leads to cell renewal, the skin takes on a healthy and fresh appearance.
Lifting effect. Skin cells are renewed and saturated with moisture, the oval of the face is tightened. Fights hyperpigmentation. The stratum corneum is exfoliated, skin cells are renewed, and facial tone is evened out.
Introduction
Despite the achievements of modern medicine, uncompensated blood loss remains the leading cause of death in patients with abdominal trauma [1, 11, 15, 16]. The tactics for compensating blood loss are to immediately stop bleeding, replace the volume of circulating blood and oxygen transport.
3-5% of patients with ongoing bleeding who require emergency intervention require massive blood transfusion [9, 10, 12, 13, 16]. The negative consequences of massive transfusion of allogeneic blood are well known [8-11, 13, 14, 16]. Note that massive blood transfusion also stimulates a systemic inflammatory response and significantly increases the incidence of purulent-septic complications in the postoperative period. The need for donor blood components makes it possible to reduce alternative methods, especially in situations in which it is impossible to refuse or limit blood transfusion [8, 10]. The use of autologous blood components is a common and safe alternative to donor blood, which has become widespread in elective surgery. In emergency surgery, when preoperative preparation is impossible, this problem can be solved by intraoperative reinfusion of spilled blood with Cell Saver devices [1-7, 8, 10]. Despite the existing positive experience, the methodological basis and material and technical equipment of hospitals limit the use of this method in emergency surgery.
The purpose of the study is to compare the effectiveness of intraoperative transfusion of donor and own red blood cells in patients with diseases and injuries accompanied by blood loss of more than 70% of the circulating blood volume.
Material and methods
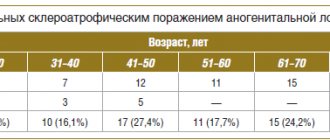
A retrospective analysis of the course of anesthesia and the results of treatment of 91 patients with injuries and diseases complicated by intra-abdominal bleeding in a volume of more than 4 liters was carried out (Table 1).
All patients were operated on as an emergency to stop bleeding. Anesthesia was standard (ketamine, fentanyl, Nimbex in recommended doses). Artificial ventilation of the lungs was carried out in the normal ventilation mode with an air-oxygen mixture (FiO2 1.0). Intraoperative infusion-transfusion therapy (ITT) included colloid and crystalloid media, fresh frozen plasma, donor erythrocytes (DE) and autoerythrocytes (AE), obtained using intraoperative hardware reinfusion of shed blood on a continuous autotransfusion system CATS (Fresenius). ITT tactics were based on determining the volume of blood loss and differed at the stages of surgical intervention. Before stopping bleeding, solutions were predominantly used under the control of hemodynamic parameters (ratio of colloids to crystalloids 2:1). The indication for DE transfusion before bleeding stopped was a hemoglobin level of less than 90 g/l. To support hemodynamics when a high infusion rate was ineffective, sympathomimetics were prescribed (dopamine at a dose of 2 mcg/kg min, adrenaline at a dose of 0.1 mcg/kg min). Lost red blood cells and blood coagulation factors were compensated during bleeding control, continuing after surgical hemostasis was achieved. Correction of electrolyte disturbances and metabolic disorders was carried out throughout the intervention.
Depending on the transfusion media used, all patients were divided into 2 groups. Group 1 included 14 patients (9 men and 5 women), in whom DE was predominantly used (more than 60% of the total volume of blood transfusion). The severity of the injury on the ISS scale was 54.5±3.4 points. The average age of patients was 47±4.9 years. Globular volume deficit 55.9±4.1%. The 2nd group included 77 people (73 men and 4 women), in whom AEs returned using hardware reinfusion were predominantly used (more than 60% of the total volume of blood transfusion). The severity of the injury on the ISS scale was 44.1±3.2 points. The average age of patients was 43±2.1 years. Red blood cell deficiency 56.9±1.9%. In terms of age, severity of injury, volume of recorded blood loss, and red blood cell deficiency, the differences in the groups were statistically insignificant.
The immediate results of intraoperative blood transfusion were assessed by indicators of red blood (Hb, Ht), oxygen content in arterial (CaO2) and mixed venous (CvO2) blood, hemoglobin saturation in mixed venous blood with oxygen (SvO2), tissue oxygen extraction ratio (O2ER) and the dynamics of the content lactate (Lac). Mortality was analyzed - in-hospital, intraoperative and postoperative.
Statistical processing of the material was carried out using the statistical package IBM SPSS Statistics 19 (IBM Inc., USA). The average values (M), standard deviation (σ), and standard error of the mean (s) were determined. For intergroup comparison, the Mann-Whitney, Kruskal-Wallis, Friedman, and Wilcoxon tests were used. The relationship between variables was determined using the test (χ2), odds ratio (OR), Pearson correlation coefficient (R), Kendall (τ) and Spearman (ρ). Data are presented as M±s. P=0.05 was taken as the threshold level of statistical significance.
Results and discussion
In group 1, during the operation (182±19 minutes), 9503±1056 ml of infusion and transfusion media were administered to patients. Before surgical stop of bleeding (124±11 minutes from the start of the operation), 75±6.7% of the total volume of ITT was transfused. In total, during the operation, due to DE and AE, 54.4 ± 13.1% of lost erythrocytes were returned. In group 2, during 180±8.5 minutes of surgery, the volume of ITT was 11,258±547 ml, including 83±2.1% of the total intraoperative volume of ITT was used before surgical hemostasis (after 141±9 minutes). During surgery, 72.5±4.4% of lost red blood cells were returned. Differences in the volume and structure of infusion therapy between groups were statistically insignificant; the structure of ITT is presented in Table. 2.
There was a statistically significant difference in absolute blood transfusion volumes between groups. Thus, the volume of media containing erythrocytes was significantly less in group 1 (p = 0.008), therefore, with the predominant use of DE, it was possible to return fewer erythrocytes than with the combined use of AE and DE.
Hb and Ht levels are often used as reference indicators when assessing the results of blood transfusion. We found a statistically significant increase in Hb and Ht after blood transfusion in each group (Table 3),
however, these indicators were significantly lower than the reference values. There were also inverse relationships between the increase in Hb and Ht parameters after blood transfusion and the volume of used media containing erythrocytes - for Hb τ = –0.285 (p = 0.002), for Ht τ = –0.256 (p = 0.001). There was an inverse relationship between the volume of ITT and the levels of Hb at the end of surgery (τ= –0.225, p=0.004) and Ht at the end of surgery (τ= –0.221, p=0.004). With a volume infusion necessary to replenish the circulating blood volume and support, due to hemodilution, the need for blood transfusion supposedly increases (partial correlation coefficient adjusted for the volume of blood loss R = 0.529, p < 0.0001).
Blood transfusion is primarily aimed at compensating for lost oxygen carriers and, accordingly, CaO2 should increase after it. However, in both groups we did not detect significant dynamics of CaO2 after blood transfusion (see Table 2). In addition, the correlations between CaO2 at the end of surgery and the volume and quality of returned red blood cells were not statistically significant. Accordingly, intraoperative blood transfusion was not accompanied by an immediate increase in CaO2, and hemic disturbances in oxygen transport persisted immediately after blood transfusion.
The central place in the pathophysiology of any critical condition is occupied by oxygen debt, which directly affects the survival of patients and the development of complications. In the clinic, it is determined by indirect signs - CvO2, SvO2, Lac, O2ER. At all stages of the study, in both groups, CvO2 was below normal values, without significantly differing between groups and at intervention stages within the group (see Table 2). Of all the correlations between CvO2 and ITT characteristics, only the connection with the degree of compensation for blood loss (percentage of returned red blood cells compared to lost ones) had statistical significance - τ = 0.223 (p = 0.038).
SvO2 also did not differ significantly between groups or within each group during the study phases. Its average values remained reduced and depended on the volume of blood transfusion (τ=0.197, p=0.046) and the volume of ITT (τ= –0.197, p=0.046).
O2ER exceeded expected values at all stages of the study. In group 1 it changed insignificantly. In group 2, there was a tendency toward normalization of O2ER after ITT (see Table 2), when it decreased by 26.2% (p>0.05). At the end of the operation, O2ER in both groups exceeded normal values and depended on red blood cell deficiency (τ=0.205, p=0.04), the volume of intraoperative ITT (τ=0.197, p=0.046) and the degree of compensation for lost red blood cells, expressed as a percentage (τ = –0.197, p=0.046).
In both groups, mean Lac values increased during surgery (see Table 3). In the 1st group, the Lac content after stopping bleeding increased by 37% (by 3.06±0.50 mmol/l; p<0.0001), in the 2nd group - by 43.6% (by 2.80 ±0.39 mmol/l; p=0.015). In group 2 at the end of the operation, the Lac level was significantly lower than in group 1 (p = 0.032). When analyzing the correlations between Lac at the end of the operation and ITT characteristics, there was a statistically significant relationship with the volume of DE used (τ=0.428, p<0.0001), the proportion of DE in the structure of blood transfusion (τ=0.309, p=0.0003), and the volume of blood transfusion (τ=0.242, p=0.004), volume of ITT (τ=0.253, p=0.003). Of course, the Lac level increased at the end of the operation for many reasons, and the identified associations reflected only the contribution of some characteristics of intensive care.
So, intraoperative transfusion of large volumes of AE and DE and replacement of circulating blood volume did not lead to immediate restoration of the hemic component of oxygen transport and compensation of oxygen debt.
When analyzing mortality rates, we found significant differences between groups. Thus, in the 1st group, 12 (85.7%) patients died, in the 2nd group - 45 (58.4%) patients (p = 0.039). Intraoperative mortality did not differ between groups. In group 1, 5 (35.7%) patients died on the operating table; in group 2, 28 (36.4%) patients died. Postoperative mortality was significantly lower in group 2. In the 1st group, 7 (50%) people underwent surgery and died in the postoperative period (on the 1st day - 21.4%, after 3 days - 28.6%), in the 2nd group - 17 (22% ) patients (on the 1st day - 14.3%, on the 2-3rd day - 1.3%, later than 3 days - 6.4%) (p = 0.018).
Statistically significant differences in mortality between groups were also reflected in the odds ratio (OR) of survival (“survived/died”). In group 1, this figure was 0.403 (95% confidence interval - CI = 0.183-0.885; p = 0.011), in group 2 - 1.385 (95% CI = 1.101-1.741). Consequently, predominantly intraoperative DE transfusion increases the risk of death in the postoperative period.
Of course, intraoperative factors influencing mortality in acute blood loss require further study, including a detailed analysis of the features of intraoperative ITT.
Thus, with the predominantly intraoperative use of autoerythrocytes to compensate for blood loss of more than 70% of the circulating blood volume, hospital and postoperative mortality is reduced.
With maximum intraoperative use of autologous blood, there is less accumulation of lactate at the end of the operation.
In case of acute blood loss of more than 70% of the circulating blood volume, intraoperative hemotransfusion of autoerythrocytes and donor erythrocytes in any ratio is not accompanied by an immediate increase in hemoglobin and hematocrit, normalization of oxygen balance (oxygen content in arterial and mixed venous blood, hemoglobin saturation in mixed venous blood with oxygen, oxygen extraction coefficient ).