Osteoarthritis problem
The problem of osteoarthritis in the Russian Federation is one of the most pressing, occupying a leading position among all diseases of the musculoskeletal system in terms of prevalence.
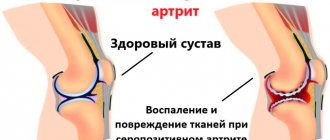
Epidemiological studies conducted over the past 3-5 years show that verified osteoarthritis by clinical and radiological manifestations is present in no less than 13% of the population of various ages, and its prevalence increases significantly in the elderly. This disease negatively affects performance, social activity, and self-care at home, significantly worsening the quality of life. Previously, osteoarthritis was called a degenerative disease; now, according to leading experts, it is a chronic inflammatory process that affects not only cartilage tissue, but also the joint capsule, tendons, ligaments, and subchondral bone. Of particular interest is damage to the shoulder joints, and it should be noted that the opinion on the etiopathogenesis and clinical manifestations of this pathology is ambiguous.
Currently, the definition of “humeroscapular periarthritis” is not used; in our study, in accordance with the modern classification, patients with the following nosology related to periarticular lesions of the shoulder joints were included:
- Tendinitis of the rotator cuff muscles and bicipital tendinitis M 75.2 ;
- Calcific tendinitis M 75.3 ;
- Subacromial (impigment) collision syndrome M 75.1 ;
- Retractile capsulitis M 75.0 ;
Purpose of the study: to evaluate the effectiveness, safety and tolerability of the drugs “Artradol” and “Artracam” in patients with periarticular lesions of the shoulder joints (humeral periarthritis).
Materials and methods: the study included 120 patients with periarticular lesions of the shoulder joints, namely:
- Rotator cuff tendinitis and bicipital tendinitis – 34 people
- Calcific tendinitis – 38 people
- Subacromial (impigment) impingement syndrome – 27 people
- Retractile capsulitis – 21 people
We observed over time 47 (39.17%) men and 73 (60.83%) women, whose average age was 54.5 ± 8.37 years in the range from 45 to 75 years. There were 32 (26.66%) working in observation groups. There are 11 (9.17%) unemployed people of working age, 77 (64.17%) pensioners.
In the first (control) observation group, no chondroprotective drugs were prescribed, including Artradol and Artracam.
In the second group, every other day Artradol was administered intramuscularly, the dry substance was dissolved in 1 ml of water for injection. The first three injections contained a dose of 0.1 g, starting with the fourth injection, the dose increased to 0.2 g.
In the third group, Artracam was prescribed as a chondroprotector; the contents of one sachet were dissolved by patients in 200 ml of water and taken orally once a day for 6 weeks.
In the fourth group, a combined prescription of “Artradol” and “Artracam” was carried out according to the scheme of alternating intramuscular administration of “Artradol” 0.2 mg and “Artracam” 1 sachet orally every other day.
In the study, 35 0.1 mg ampoules of Artradol and 20 sachets of Artracam were prescribed per course of treatment per patient.
The study included patients with periarticular lesions of the shoulder joints (diagnosis of glenohumeral periartitis according to the ACR criteria of 1987). They had radiographic stage II or III of osteoarthritis of the acromioclavicular joint according to Kellgren-Lawrence. All patients signed informed consent.
The initial exclusion criteria were patients with secondary gonarthrosis, infectious arthritis, systemic inflammatory diseases, gout, pseudogout, Paget's disease, intra-articular fractures, ochranosis, acromegaly, hemochromatosis, Wilson's disease, primary chondromatosis, chondrocalcinosis, concomitant severe diseases (uncontrolled arterial hypertension, unstable angina pectoris, cardiovascular failure, diabetes mellitus type 1. Severe liver and kidney diseases), with a stomach or duodenal ulcer within the last month, with bleeding or a tendency to bleeding, with a history of thrombophlebitis, pregnancy, lactation, as well as body mass index more than 40 kg/m2. Also, individuals included in the study were not required to undergo intra-articular injections of any drugs within 6 weeks before the start of the study. Patients with known hypersensitivity to chondroitin sulfate or glucosamine sulfate were excluded.
The observed patients had a pronounced need to take non-steroidal anti-inflammatory drugs (NSAIDs) (within 30 days over the last 3 months). At the beginning of the study, NSAIDs were not discontinued; patients continued taking this group of drugs in accordance with the individual presence of contraindications, followed by a dose reduction or discontinuation of NSAIDs based on their well-being. So, they took: Nimesulide in a daily dose of 200 mg in the form of sachets or Nise tablets, Ketoprofen intramuscularly 100 mg or 2 capsules (200 mg) per day, Meloxicam tablets or injections 15 mg per day, Ibuprofen 1200 mg-2400 mg in capsules per day, Airtal 1 tablet 2 times a day or 1 sachet 2 times a day, Naysilat 600 mg 2 times a day in the form of tablets, strictly 1 hour before meals, Ibuklin 1 tablet 3 times a day before meals or 2-3 hours after food without chewing.
Assessing the need for NSAIDs, we emphasize that 97.5% of the patients included in the study took NSAIDs regularly. As mentioned above, the personalized selection of NSAIDs was controlled, taking into account contraindications and predicted unwanted side effects. The optimal dose of NSAIDs was selected, since in more than 13.33% it was unreasonably overestimated or underestimated.
During the therapy period, intra-articular injections of glucocorticoids, hyaluronic acid preparations and any other drugs were not performed. Also excluded from the list of prescriptions were drugs that have chondroprotective properties, such as anticoagulants, antiplatelet agents, fibrinolytics, and physiotherapy procedures were not performed.
Patients included in the study suffered from comorbid diseases. We identified 58 patients with verified nosologies, which accounted for 48.3% of all examined. Note that hypertension was diagnosed in 51 (42.5%), spondyloarthrosis of the thoracic and lumbosacral spine - in 29 (24.2%), atherosclerosis - in 21 (17.5%), discirculatory encephalopathy 2 - in 4 (3.3%), coronary heart disease – in 3 (2.5%), chronic obstructive bronchitis – 2 (1.6%), chronic cholecystitis outside the acute stage – 2 (1.6%), duodenal ulcer (history of exacerbations 1 and 2 years ago, respectively) - 2 (1.6%), chronic pyelonephritis, chronic renal failure stage I - 1 (0.8%), type 2 diabetes mellitus in the compensated stage - 1 (0. 8%). In 62 patients, comorbid conditions were not diagnosed, which amounted to 51.6%.
Patients were prescribed supportive pharmacotherapy for concomitant pathology: diuretics in combination with ACE inhibitors, as well as beta-blockers for the treatment of hypertension, NSAIDs and chondroprotectors for the treatment of spondyloarthrosis, statins for the correction of dyslipidemia, nootropics for patients with dyscirculatory encephalopathy, choleretic drugs and hepatoprotectors - for patients with chronic cholecystitis, proton pump inhibitors - for patients with peptic ulcer disease. Treatment of comorbid conditions was coordinated with specialists in the profile of concomitant pathology.
In the observed patients, initially, using a visual analog scale (VAS, mm), pain at night in bed, pain while sitting or lying down, pain in an upright position, pain when moving the upper limbs (active and passive) and pain on palpation were determined.
Table 1 Initial parameters of pain syndrome in observed patients (n=120)
| Characteristics of pain syndrome | YOUR, mm |
| Pain in bed at night | 41,5±3,82 |
| Pain while sitting and lying down | 43,2±4,03 |
| Pain in an upright position | 45,4±4,12 |
| Pain with active movements of the upper limbs | 46,3±4,67 |
| Pain with passive movements of the upper limbs | 49,7±5,11 |
| Pain on palpation | 53,9±4,96 |
Table 2 Initial severity of pain syndrome in observation groups
| Characteristics of pain syndrome | 1st group | 2nd group | 3rd group | 4th group |
| Pain in bed at night | 41,5±3,82 | 41,2±2,63 | 40,6±3,15 | 41,2±3,87 |
| Pain while sitting and lying down | 43,2±4,03 | 44,3±3,84 | 42,3±2,90 | 43,8±3,97 |
| Pain in an upright position | 45,4±4,12 | 46,2±3,76 | 45,7±3,42 | 45,8±3,52 |
| Pain with active movements of the upper limbs | 46,3±4,67 | 47,1±4,85 | 46,4±4,71 | 46,7±4,72 |
| Pain with passive movements of the upper limbs | 49,7±5,11 | 50,2±4,98 | 49,8±5,09 | 50,1±5,13 |
| Pain on palpation | 53,9±4,96 | 54,6±4,87 | 54,2±4,93 | 54,4±4,63 |
Table 3 Initial parameters of the WOMAC questionnaire in observed patients (n=120)
| Sections of the WOMAC Questionnaire | Points (average values) |
| Section A. Pain | 43,8±4,09 |
| Section B. Stiffness | 26,2±3,91 |
| Section B. Difficulties in daily activities | 53,52±4,87 |
Table 4 Initial expression of WOMAC questionnaire parameters in observation groups
| 1st group | 2nd group | 3rd group | 4th group | |
| Section A. Pain | 42,6±3,57 | 43,5±4,01 | 44,8±4,16 | 44,5±3,94 |
| Section B. Stiffness | 25,4±3,25 | 26,7±3,84 | 28,3±4,06 | 27,9±4,11 |
| Section B. Difficulties in daily activities | 52,5±4,62 | 54,1±4,48 | 55,3±4,69 | 53,6±4,37 |
Initially, statistically significant differences in the parameters presented in table. 2 and table. 4 were not observed in the observation groups.
To quantitatively assess coordination in the observation groups, we conducted the “Get Up and Walk” test, which determined the ability to get up from a chair without support within an estimated time in seconds, as well as on a numerical rating scale (NRS) in points.
Table 5 Initial parameters of the “Get up and go” test in observation groups
| 1st group | 2nd group | 3rd group | 4th group | |
| Inability to get up from a chair without support (sec) | 9,1±3,71 | 8,9±4,24 | 8,1±3,55 | 9,0±4,05 |
| Inability to get up from a chair without support (CHS points) | 9,2±3,83 | 8,5±3,96 | 8,0±3,37 | 8,8±3,67 |
Safety assessment was carried out according to the following indicators: frequency and nature of adverse events that developed during the observation period, their relationship with the study drug (no connection, unlikely, possible, probable, definite, unknown).
An adverse event was considered to be any medical event that occurred to the patient during participation in the study, regardless of connection with the therapy. The criterion for early exclusion of a patient from their study was the development of serious adverse events (events leading to death, threatening the patient’s life, and also requiring surgical intervention and additional therapy). All cases of adverse events were recorded in the study chart and primary documentation, and were also sent to the study sponsor.
During the study, blood test parameters were monitored: clinical (hemoglobin, leukocytes, ESR) and biochemical (AST, ALT), urine analysis.
During the open study, patients were excluded according to the following criteria: ineffectiveness of therapy for the underlying disease - persistence or worsening of pain requiring treatment adjustment, serious adverse events, refusal to participate in the study, as well as violation of the protocol.
Artradol (chondroitin sulfate) – a new drug in the treatment of osteoarthritis
The causes of the development of OA are various genetic (in patients with congenital diseases of bones and joints; OA is much more common in women than in men) and acquired (old age, excess weight, metabolic disorders, previous joint surgeries, excessive loads - for example, ballet dancers, loaders, or joint injuries) factors. Colds have a certain significance (for example, arthrosis in workers in hot shops). Causal factors for OA are presented in Table 1. With age, the risk of developing OA increases significantly. Symptoms of OA are often detected as early as 30–40 years of age; over the age of 50, they occur in 27% of people; after 60 years, almost everyone suffers from this disease. The most common OA joints of the lower half of the body (hip, knee, first metatarsophalangeal), as well as lesions of the spine. Over time, deforming arthrosis develops; due to curvature, the affected areas can take on bizarre shapes: “swan neck”, “button loop”. Degenerative-dystrophic diseases of cartilage tissue are one of the main causes of chronic pain joint syndrome [1,4]. At the beginning of the disease, joint pain is practically absent at rest, but appears when the joint is loaded. In addition to pain, there is a crunch in the joint; over time, stiffness and a decrease in range of motion occur. Advanced arthrosis can lead to disability. Diagnosis of primary OA does not cause any difficulties for doctors. It is based on the American College of Rheumatology (ACR) diagnostic criteria, which have not changed significantly over the past 20 years. These criteria require the presence of clinical manifestations of OA and specific radiological signs. X-ray of joints is not only an important tool for diagnosing OA, but also allows a series of radiographs to make a long-term prognosis of the disease, judge the effectiveness of treatment and determine the tactics of patient management during planned surgical interventions (endoprosthetics of large joints) [2]. The choice of treatment for OA is determined by a number of factors, among which the main ones are: the severity of pain and inflammatory response, functional failure, the degree of structural changes, age and the presence of concomitant diseases [1]. As a rule, an integrated approach is used in the treatment of OA using various non-drug methods, pharmacotherapy, and in some cases, surgical intervention (arthrodesis, arthroplasty). Among the non-drug methods of treating OA, physiotherapy (thermal procedures, ultrasound) is widely used. For OA, diadynamic currents and electrophoresis with painkillers and anti-inflammatory drugs are also prescribed. Treatment with mud rich in microelements is very effective. Applications of paraffin with ozokerite and radon baths also help. For OA, massage can be helpful, but you should avoid direct impact on the diseased joint, as this can increase the inflammatory response in it. Patients should be explained that sharp temperature fluctuations (high temperatures, cold exposure) are contraindicated in OA. In summer, as a rule, the disease worsens in those who spend a lot of time on the beach, and in winter, “walruses” are especially susceptible to it. In autumn, it is important to prevent hypothermia. Therapeutic gymnastics are used effectively for OA, dancing, fast ones at that, is very useful, waltzing for the elderly, cycling, swimming, and walking long distances are also useful. The diet for OA should contain a sufficient amount of proteins and vitamins (especially C, D). Vitamin D is found in optimal quantities in cod and tuna liver, as well as in milk, sour cream, yoghurt and cream. It is recommended to include in your diet more often all kinds of salads dressed with vegetable oil, nuts, honey and cranberries. For dessert, apricots, apples, plums, cherries, and cherries are healthy. It is important to correct weight; prevention of bruises and injuries is necessary. Patients with OA are recommended to wear comfortable leather shoes with low heels and light clothing made from natural fabrics that do not restrict movement. The use of orthoses significantly reduces the load on the affected joints, reducing pain. In folk medicine, the choice of medicinal plants for OA is quite wide: these are wild rosemary, birch leaves and buds, black elderberry flowers, cherry fruits, sweet clover, spruce and juniper branches for baths, cabbage leaves, marsh marigold, nettle, sea buckthorn, tansy, purslane , thyme, anemone, oak ragwort, bathwort, buttercups, cold wormwood, lumbago, rhododendron. However, the evidence base for the effectiveness of these drugs and the ability to solve problems arising from OA only with their use are currently lacking. It is clear that many patients with OA do not receive adequate medical care. A UK study found that only 33% of patients over 50 years of age who had experienced knee pain for 1 year or more visited a GP. The remaining patients self-medicated [2]. For a long time, analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs) were used as drugs for the treatment of OA. However, a large number of side effects, primarily from the gastrointestinal tract (GIT) and cardiovascular system, limits their widespread use, especially in older age groups [2,16]. Russian, European and American guidelines recommend the use of NSAIDs for OA in minimal effective doses and, if possible, short courses [1,3,42]. In 2003, the so-called SYmptomatic Slow Acting Drugs for OsteoArthritis (SYSADOA) were included in the recommendations of the European League Against Rheumatism EULAR for the management of patients with OA. A typical representative of SYSADOA is chondroitin sulfate (CS). The OA Research Society International (OARSI) and EULAR have included cholesterol in recommendations for the management of patients with OA. CS is the main component of proteoglycans, which together with collagen fibers make up the cartilage matrix; it is part of bone, cartilage, tendons, and ligaments, performing a number of important metabolic and biomechanical functions. The cholesterol molecule is a sulfated glycosaminoglycan, which consists of long unbranched chains with repeating N-acetylgalactosamine and glucuronic acid residues. Most N-acetylgalactosamine residues are sulfated at the 4th and 6th positions. This structure of the cholesterol molecule determines its participation in the processes of transport of water, amino acids and lipids in the avascular areas of cartilage. Long chains of cholesterol, which are part of the extracellular matrix, determine the most important biomechanical properties of cartilage tissue [1,4,36]. The unique features of the cholesterol molecule formed the basis for its use in OA [1,4,15]. CS has been used in medical practice for more than 40 years; the main source of its production is the bovine trachea. The raw material is a mixture of cholesterol molecules of various weights and lengths with variations in the position of sulfate groups. The drugs produced have varying degrees of purification from impurities. All these differences may ultimately lead to different effects of cholesterol, which should be taken into account when prescribing original drugs or generics [1,4,20,39]. When taken orally, cholesterol is quickly adsorbed from the gastrointestinal tract, while predominantly low-molecular-weight derivatives (up to 90% of the dose taken) and only 10% of native molecules enter the systemic circulation. The bioavailability of cholesterol depends on the molecular weight, degree of sulfation, and the presence of impurities and averages from 10 to 20% [1.39]. The maximum concentration of cholesterol in the blood is reached 3–4 hours after administration, and in the synovial fluid – after 4–5 hours. The drug is excreted mainly by the kidneys. A necessary condition for the effectiveness of cholesterol is its accumulation in the tissues of the joint. Thus, F. Ronca et al. [35], using radioactive tracers, revealed increased accumulation of cholesterol in both cartilage and synovial fluid. A stable concentration of cholesterol in the systemic circulation is achieved after 3–4 days, but it may take 8 to 12 weeks for the clinical effect to develop. therapy [1]. The mechanism of action of cholesterol is complex, multifaceted and covers almost all key aspects of the pathogenesis of OA. The concept of using cholesterol in the treatment of OA arose more than 30 years ago and assumed an effect on degenerative processes in cartilage tissue. CS was considered rather as a “building material” for damaged cartilage, and an increase in the concentration of cholesterol in the extracellular matrix should have contributed to the launch of reparative processes. Indeed, already in early studies the anabolic properties of cholesterol were identified: an increase in the synthesis of type II collagen and proteoglycans, which was confirmed by later studies [1,28,40]. It has also been shown that cholesterol can increase the synthesis of hyaluronic acid by synovial cells [1,38]. In subsequent studies of the pathogenesis of OA, extensive material was obtained indicating the involvement in the pathological process of not only articular cartilage, but also subchondral bone, synovial membrane, joint capsule and periarticular tissues. It is no coincidence that OA is currently considered an “organ disease” accompanied by persistent inflammation [1,30]. Upon further study of the mechanisms of action of cholesterol, it turned out that the drug has a wide range of biological reactions and affects a variety of parts of the pathogenesis of OA. A number of studies have found an anti-inflammatory effect of cholesterol. Thus, during chronic induced arthritis in rabbits, cholesterol decreased gene expression and synthesis of cyclooxygenase-2 (COX-2), chemokine ligand 2 (C-Cmotive) in the synovial membrane. There was a decrease in the infiltration of inflammatory cells in the synovial membrane and the degree of its proliferation [1,26]. Increased synthesis of IL-1β and TNF-α is observed in all patients with OA, which is of great importance for the development and maintenance of the inflammatory process. IL-1β together with TNF-α induce the formation of such pro-inflammatory mediators as IL-8 and -6, matrix metalloproteinases (MMP), COX-2, nitric oxide, prostaglandin E2, and have a catabolic effect on the metabolic processes of cartilage tissue [1,34 ]. Some in vivo studies have found that cholesterol can reduce the concentration in joint tissues of IL-1 β [1,34] and other pro-inflammatory mediators (IL-6, nitrogen nitrite synthase, prostaglandin E2) [1,8,11,12] . The most important role in the metabolic processes of cartilage tissue belongs to the family of extracellular endopeptidases (MMPs), capable of destroying all types of extracellular matrix proteins. Native collagen is destroyed predominantly by MMP-1, -8 and -13, and the latter occupies a special place, since it is involved specifically in the destruction of type 2 collagen [1,22]. The main role in the pathogenesis of OA is played by MMP-3, a key link in the homeostasis of articular proteoglycans, and MMP-9, which takes an active part in inflammatory reactions, degradation of bone and cartilage tissue. The degree of damage to joint tissue in OA may decrease due to the activity of MMPs. A number of studies have shown that cholesterol can suppress the induced expression of MMP-13 in chondrocytes [1,19]. The use of cholesterol in experimental models has also demonstrated effective inhibition of MMP-3 and -9 [1,13,33]. CS has antioxidant properties, protecting cells by inhibiting protein oxidation reactions, lipid peroxidation and suppressing the formation of free radicals. Some studies have found that cholesterol suppresses chondrocyte apoptosis [1,9,10,25]. It is known that activation of nuclear factor κβ (NFκβ) is a key link in the development of inflammatory and immune reactions. The binding of NFκβ to the promoter of the corresponding gene increases the expression of proinflammatory cytokines, activates nitric oxide synthase, COX-2, phospholipase A2, MMPs involved in inflammatory reactions and tissue damage. CS reduces the activation of NFκβ and its nuclear translocation in chondrocytes and synovial membrane cells, which may explain some therapeutic aspects of the use of this drug in OA. It is believed that cholesterol can cause a similar effect in macrophages and hepatocytes, and this opens up new opportunities for its use in diseases accompanied by high inflammatory activity. According to some researchers [1,37], the use of cholesterol is promising for inflammatory bowel diseases, atherosclerosis, Parkinson's disease, Alzheimer's disease, multiple sclerosis, amyotrophic lateral sclerosis, rheumatoid arthritis and systemic lupus erythematosus. There are indications of the possible effectiveness of cholesterol in psoriasis [1,32]. CS reduces resorption processes by suppressing the expression of RANKL and activating the synthesis of osteoprotegerin, thus influencing the processes of subchondral bone remodeling [1,24]. The ability of cholesterol to reduce the severity of inflammatory reactions, reduce pain and improve functional indicators has been studied in many clinical studies. In 2000, BF Leeb et al. [1,23] conducted a meta-analysis of 7 double, placebo-controlled, parallel group studies. The effectiveness of cholesterol in 372 patients was determined using the Lequesne index, pain scores were assessed using VAS. The duration of the studies ranged from 56 to 1095 days, mostly from 90 to 180 days. A significant superiority of cholesterol compared to placebo was obtained for all indicators, including pain - 0.9 (95% CI 0.8-1.0) and effect on joint function - 0.74 (95% CI 0.65-0. 85). The authors also noted the need for further research with larger numbers of participants. In the same year, T.E. McAlindon et al. [1,29] performed a meta-analysis of 15 double-blind placebo-controlled studies of the effectiveness of glucosamine (GA) and cholesterol (6 - GA and 9 - CS) as symptomatic agents (pain reduction and improvement of functional status) for treatment for > 4 weeks. OA of the knee and hip joints. The authors assessed the strength of the effect (standardized mean difference): <0.2 – small effect, 0.5 – moderate and >0.8 – significant. The analysis found that the overall effect size for cholesterol was 0.78 (95% CI 0.6–0.95), but the effect size was reduced when only large-scale or high-quality studies were considered. G. Bana et al. [1,6] analyzed the results of 7 randomized clinical trials (RCTs), which showed a significant reduction in pain intensity and Lequesne index with the use of cholesterol for OA of the hip and knee joints. Given the emergence of new large studies and controversial opinions about the use of slow-acting drugs for the treatment of OA, experts from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) decided to prepare their own recommendations on the use of slow-acting drugs for OA [1,7]. All conclusions were based on the use of the GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology: a consistent assessment of the quality of evidence was carried out, taking into account the balance between the advantages (reduction of pain, improvement of functional indicators) and disadvantages of the drug (adverse events (AE)) with subsequent determination of the strength recommendations. The analysis included data from RCTs and meta-analyses from 1950 to 2007. It was found that the advantages of using cholesterol outweighed its disadvantages (AEs) and this drug can be recommended as an effective treatment for OA. To evaluate the symptom-modifying effect, a multicenter, double-blind RCT of the efficacy and safety of cholesterol, GA, celecoxib, and a combination of cholesterol and GA in patients with knee OA was conducted in the United States [1,14]. 1583 patients with knee OA (radiographic stage II–III) and pain lasting >6 months were randomized. Patients took standard doses of drugs: 1500 mg/day. GA, 1200 mg/day. CS, combination of CS and GA, celecoxib 200 mg/day. or placebo for 24 weeks. The primary endpoint was a 20% reduction in joint pain at the end of the study. The authors generally did not reveal a significant effect of cholesterol and GA on pain scores (the effect was considered significant when its intensity decreased by 20%) compared to placebo. However, the combined use of cholesterol and GA in patients with moderate and severe pain was more effective than placebo (79.2% versus 54.3%, respectively, p = 0.002). In addition, cholesterol significantly reduced joint swelling (p<0.013). The structure-modifying effect of these drugs could not be detected. There is also evidence of the positive effect of cholesterol in OA of the joints of the hands. C. Gabay et al. [1,17] conducted an RCT that included 162 patients with manifest OA of the hand joints. Study participants received either 800 mg cholesterol (n=80) or placebo (n=82) for 6 months. When analyzing the data, it was revealed that in the cholesterol group there was a more pronounced decrease in pain intensity (general assessment by the patient) compared with placebo (differences in VAS -8.7 mm, p = 0.016), better indicators of functional status (differences in FIHOA = -2 .14; p=0.008) and shorter duration of morning stiffness. The need for acetaminophen and the frequency of AEs did not differ between groups. Thus, most studies have found the effect of cholesterol on the main clinical manifestations of OA. The structure-modifying effect of cholesterol has been studied in several RCTs. The effect of cholesterol on the dynamics of radiological narrowing of the joint space, which is an indirect marker of articular cartilage damage in OA, was assessed. V.A. Michel et al. [1,31] conducted an RCT that included 300 patients with knee OA who received cholesterol at a dose of 800 mg/day. for 2 years. A significant stabilizing effect of cholesterol on the width of the joint space was revealed. An international RCT (622 patients) also found a significant protective effect of cholesterol on changes in the width of the joint space in patients with OA of the knee joints [1,21]. At the same time, a meta-analysis of 3 RCTs lasting up to 2 years conducted in 2010 [1,18] showed a statistically significant, but clinically small decrease in the rate of joint space narrowing - 0.13 mm (95% CI 0.06–0.19, p=0.002), which corresponded to an effect size of 0.23 (95% CI 0.11–0.35, p=0.001). Similar results were obtained by Y.N. Lee et al. [1,27] during a meta-analysis of 6 RCTs (2 – GA and 4 – CS, 1502 patients). A significant effect was observed after 2 years of CS treatment and 3 years of GA treatment. The authors noted a small but statistically significant protective effect of GA and cholesterol on the radiographic progression of knee OA, amounting to 0.261 for cholesterol (95% CI 0.131–0.392, p<0.001). The use of magnetic resonance imaging (MRI) allows us to obtain additional information about the joints, including the volume of articular cartilage, the condition of the subchondral bone, menisci, and synovium. Thus, LM Wildi et al. used MRI to assess the structure-modifying effect of cholesterol [1,41]. With support from the National Institutes of Health of Canada, a one-year pilot multicenter RCT was conducted that included 69 patients with knee OA and signs of synovitis. Already after 6 months. in the group taking cholesterol at a standard dose of 800 mg/day. There was less loss of total cartilage volume (p=0.03), lateral cartilage (p=0.015) and tibial cartilage (p=0.002), and similar results were maintained throughout the observation period. The authors also noted a lower degree of change in the subchondral bone in the study group compared to the control group. The differences reached statistical significance 1 year after the start of the study and were predominantly observed in the lateral parts of the joint. Thus, in the literature there are indications of the structure-modifying effect of cholesterol in OA. Further studies using newer methods are needed to confirm this effect and determine its clinical significance. XC has a high safety profile. None of the clinical studies revealed significant side effects of cholesterol, including long-term treatment. The metabolism of cholesterol occurs without the participation of the cytochrome P 450 system. Consequently, the risk of adverse reactions when combined with cholesterol with other drugs is extremely small. This is very important for patients of older age groups, who have high comorbidity and, consequently, the need for simultaneous use of a large number of medications. Taking into account the data from clinical and laboratory studies, EULAR considers cholesterol as one of the safest drugs for the treatment of OA [1]. Currently, various dosage forms of cholesterol are used in the treatment of OA. The drug Artradol, containing lyophilized CS powder for the preparation of a solution for intramuscular injection, has found widespread use. The drug is made from high-quality raw materials using modern technologies. Artradol, like other cholesterol drugs, has chondroprotective properties, suppresses the activity of enzymes that cause degradation of articular cartilage, stimulates the production of proteoglycans by chondrocytes, enhances metabolic processes in cartilage and subchondral bone, and affects phosphorus-calcium metabolism in cartilage tissue. The drug is easily absorbed when administered intramuscularly, accumulates in cartilage tissue and after 30 minutes. detected in significant concentrations in the blood after 15 minutes. – in synovial fluid. The maximum concentration in articular cartilage is achieved after 48 hours [2,4,5]. Artradol has anti-inflammatory and analgesic properties, helps reduce the release of inflammatory mediators and pain factors into the synovial fluid through synoviocytes and macrophages of the synovial membrane, and suppresses the secretion of leukotriene B4 and prostaglandin E2. The drug helps restore the cartilaginous surfaces of the joints, prevents the collapse of connective tissue, normalizes the production of joint fluid, which leads to improved joint mobility and reduced pain. Possessing structural similarities to heparin, Artradol can prevent the formation of fibrin thrombi in the synovial and subchondral microvasculature [1,2,4]. Indications for the use of Artradol are: primary arthrosis, OA with damage to large joints, intervertebral osteochondrosis. Used intramuscularly, 0.1 g every other day. Before use, the contents of the ampoule are dissolved in 1 ml of water for injection. If well tolerated, the dose is increased to 0.2 g starting from the 4th injection. The course of treatment is 25–35 injections. Repeated course – after 6 months. The duration of repeated courses of treatment is determined by the doctor. Use with caution if you are prone to bleeding, thrombophlebitis, during pregnancy and lactation. The effect of indirect anticoagulants, antiplatelet agents, and fibrinolytics may be enhanced, which requires more frequent monitoring of blood clotting parameters when used together. OA is a fairly heterogeneous disease in which the main clinical manifestations can vary widely, and patients can be assisted by doctors of various specialties (therapist, general practitioner, rheumatologist, neurologist, orthopedist-traumatologist, etc.). In such cases, maintaining continuity may be difficult. In accordance with the approved procedure for providing medical care to patients with rheumatic diseases (Order of the Ministry of Health and Social Development of the Russian Federation No. 315n dated May 4, 2010), within the framework of primary health care in outpatient settings, such care is provided by a local physician, a general practitioner (family doctor) in accordance with established standards, taking into account the recommendations of rheumatologists. Paragraph 4 of the order states that treatment of patients with OA of small, medium and large joints without synovitis and who do not require endoprosthetics is carried out by general practitioners and general practitioners, taking into account the recommendations of rheumatologists. It is important to note that when referring to a rheumatologist, local general practitioners must provide an extract from the outpatient card indicating the preliminary diagnosis, concomitant diseases and clinical manifestations of the disease, as well as available laboratory and functional test data [2]. Thus, information about new effective drugs for the treatment of OA is important not only for highly specialized doctors, but primarily for primary care physicians (therapist, general practitioner). It is planned to conduct research on Artradol in several leading rheumatology centers in Russia. Literature 1. Anikin S.G., Alekseeva L.I. Chondroitin sulfate: mechanisms of action, effectiveness and safety in the treatment of osteoarthritis // Modern Rheumatology.2012. No. 3. 2. Zotkin E.G., Shkireeva S.Yu. Features of the management of patients with osteoarthritis in primary healthcare // RMZh. 2012. No. 27. 3. Clinical recommendations. Rheumatology Ed. acad. RAMS E.L. Nasonova. M.: GEOTAR-Media, 2010. P. 326. 4. Nikishchenkova A.S., Zhulev S.N., Zhulev N.M., Ovsyannikova N.A. Artradol (chondroitin sulfate) in the treatment of osteochondrosis and spondyloarthrosis of the spine // Neurology/rheumatology. Consilium Medicum application. 2012. No. 2. 5. Folomeeva O.M., Amirdzhanova V.N., Yakusheva E.O. and others. Analysis of the structure of the XIII class of diseases // Ros. rheumatol. 1998. No. 1. P. 2–7. 6. Bana G., Jamard B., Verrouil E. et al. Chondroitin sulfate in the management of hip and knee OA: an overview // AdvPharmacol. 2006. Vol. 53. R. 507–522. 7. Bruyere O., Burlet N., Delmas PD et al. Evaluation of symptomatic slow-acting drugs in osteoarthritis using the GRADE system // BMC Musculoskelet Dis. 2008. Vol. 9. P. 165. 8. Campo GM, Avenoso A., Campo S. et al. Chondroitin sulfate: antioxidant properties and beneficial effects // Mini Rev Med Chem. 2006. Vol. 6 (12). R. 1311–1320. 9. Campo GM, Avenoso A., Campo S. et al. Glycosaminoglycans modulate inflammation and apoptosis in LPS-treated chondrocytes // J Cell Biochem. 2009. Vol. 106(1). R. 83–92. 10. Caraglia M., Beninati SD, Alessandro AM et al. Alternative therapy of earth elements increases the chondroprotective effects of chondroitin sulfate in mice // ExpMol Med. 2005. Vol. 37. R. 476–481. 11.Chan PS, Caron JP, Rosa GJ et al. Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E(2) in articular cartilage explants // OsteoarthrCartil. 2005. Vol. 13(5). R. 387–394. 12. Cho SY, Sim JS, Jeong CS et al. Effects of low molecular weight chondroitin sulfate on type II collagen–induced arthritis in DBA/1J mice // Biol Pharm Bull. 2004. Vol. 27(1). R. 47–51. 13. Chou MM, Vergnolle N, McDougall JJ et al. Effects of chondroitin and glucosamine sulfate in a dietary bar formulation on inflammation, interleukin-1beta, matrix metalloprotease-9, and cartilage damage in arthritis // ExpBiol Med (Maywood). 2005. Vol. 230(4). R. 255–262. 14. Clegg DO, Reda DJ, Harris CL et al. Glucosamine, chondroitin sulfate and the two in combination for painful knee osteoarthritis // N Engl J Med. 2006. Vol. 354(8). R. 795–808. 15. Conte A., Volpi N., Palmieri L. et al. Biochemical and pharmacological aspects of oral treatment with chondroitin sulfate // Arzneim Drug Res. 1995. Vol. 45. R. 918–925. 16. DeHaan M., Guzman J., Bayley M. et al. Knee osteoarthritis clinical practice guidelines — how are we doing? // J. Rheumatol. 2007. Vol. 34. P. 2099–2105. 17. Gabay C., Medinger–Sadowski C., Gascon D. et al. Symptomatic effects of chondroitin 4 and chondroitin 6 sulfate on hand osteoarthritis: a randomized, doubleblind, placebo–controlled clinical trial at a single center // Arthr Rheum. 2011. Vol. 63 (11). R. 3383–3391. 18. Hochberg MC Structure–modifying effects of chondroitin sulfate in knee osteoarthritis: an updated meta–analysis of randomized placebo–controlled trials of 2–year duration // OsteoarthrCartil. 2010. Vol. 18 (Suppl 1). R. 28–31. 19. Holzmann J., Brandl N., Zemann A. et al. Assorted effects of TGFbeta and chondroitin sulfate on p38 and ERK1/2 activation levels in human articular chondrocytes stimulated with LPS // OsteoarthrCartil. 2006. Vol. 14. R. 519–525. 20. Imada K, Oka H, Kawasaki D et al. Anti–arthritic action mechanism of natural chondroitin sulfate in human articular chondrocytes and synovial fibroblasts // Biol Pharm Bull. 2010. Vol. 33(3). R. 210–214. 21. Kahan A., Uebelhart D., de Vathaire F. et al. Long–term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double–blind, placebocontrolled trial // Arthr Rheum. 2009. Vol. 60(2). R. 524–533. 22. Knäuper V., Lopez–Otin C., Smith B. et al. Biochemical characterization of human collagenase-3 // J BiolChem. 1996. Vol. 271(3). R. 1544–1550. 23. Kwan Tat S., Schweitzer H., Montag K. et al. A metaanalysis of chondroitin sulfate in the treatment of osteoarthritis // J Rheum. 2000. Vol. 27(1). R. 205–211. 24. Kwan Tat S, Pelletier JP, Lajeunesse D et al. The differential expression of osteoprotegerin (OPG) and receptor of nuclear factor kB ligand (RANKL) in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells // Clin Exp Rheum. In press. 25. Jomphe C, Gabriac M, Hale TM et al. Chondroitin sulfate inhibits the nuclear translocation of nuclear factor-kappa B in interleukin-1beta-stimulated chondrocytes // Basic ClinPharmacolToxicol. 2008. Vol. 102. R. 59–65. 26. Largo R., Roman-Blas JA, Moreno-Rubio J. et al. Chondroitin sulfate improves synovitis in rabbits with chronic antigeninduced arthritis // OsteoarthrCartil. 2010. Vol. 18 (Suppl. 1). R. 17–23. 27. Lee YH, Woo JH, Choi SJ et al. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis // Rheum Int. 2010. Vol. 30(3). R. 357–363. 28. Legendre F., Bauge C., Roche R. et al. Chondroitin sulfate modulation of matrix and inflammatory gene expression in IL–1betastimulated chondrocytes–study in hypoxic alginate bead cultures // OsteoarthrCartil. 2008. Vol. 16(1). R. 105–114. 29. McAlindon TE, LaValley MP, Gulin JP et al. Glucosamine and chondroitin for the treatment of osteoarthritis: a systematic qualitative assessment and meta-analysis // JAMA. 2000. Vol. 283(11). R. 1469–1475. 30. Martel–Pelletier J., Lajeunesse D., Pelletier JP. Etiopathogenesis of osteoarthritis. In: Arthritis and Allied Conditions: A Textbook of Rheumatology. W. J. Koopman, L. W. Moreland (eds). Baltimore: Lippincott, Wikings W., 2005. pp. 2199–2296. 31. Michel B. A., Stucki G., Frey D. et al. Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: a randomized, controlled trial // Arthr Rheum. 2005. Vol. 52(3). R. 779–786. 32. Möller I., Perez M., Monfort J. et al. Effectiveness of chondroitin sulfate in patients with concomitant knee osteoarthritis and psoriasis: a randomized, double-blind, placebo-controlled study // OsteoarthrCartil. 2010. Vol. 18 (Suppl 1). R. 32–40. 33. Monfort J., Nacher M., Montell E. et al. Chondroitin sulfate and Hyaluronic acid (500–730 kDa) inhibit stromelysin–1 synthesis in human osteoarthritic chondrocytes // Drugs ExpClin Res. 2005. Vol. 31. R. 71–76. 34. Pelletier JP, Martel–Pelletier J., Abramson SB Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets // Arthr Rheum. 2001. Vol. 44 (6). R. 1237–1247. 35. Ronca F., Palmieri L., Panicucci P. et al. Anti-inflammatory activity of chondroitin sulfate. OsteoarthrCartil. 1998. Vol. 6 (Suppl. A). R. 14–21. 36. United States Senate Committee on Health, Education, Labor and Pensions, Sub committee on aging. Center for Disease Control. Washington, DC: Department of Health and Human Services, 2004. 37. Vallieres M., du Souich P. Modulation of inflammation by chondroitin sulfate // OsteoarthrCartil. 2010. Vol. 18 (Suppl. 1). R. 1–6. 38. Verbruggen G., Veys EM Influence of sulphated glycosaminoglycans upon proteoglycan metabolism of the synovial lining cells // ActaRhumBelg. 1977. Vol. 1 (12). R. 75–92. 39. Volpi N. Quality of different chondroitin sulfate preparations in relation to their therapeutic activity // J Pharm Pharmacol. 2009. Vol. 61 (10). R. 1271–1280. 40. Wang L., Wang J., Almqvist KF et al. Influence of polysulphated polysaccharides and hydrocortisone on the extracellular matrix metabolism of human articular chondrocytes in vitro // Clin Exp Rheum. 2002. Vol. 20 (5). R. 669–676. 41. Wildi LM, Raynauld JP, Martel-Pelletier J. et al. Chondroitin sulfate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomized, double–blind, placebo–controlled pilot study using MRI // AnnRheumDis. 2011. Vol. 70 (6). R. 982–989. 42. Zhang W., Nuki G., Moskowitz RW et al. OARSI recommendations for the Management of hip and knee osteoarthritis Part III: changes in evidence following systematic cumulative update of research published through January 2009 // OsteoarthrCartil. 2010. Vol. 18 (4). R. 476–499.
Research results
Based on the results of observation of groups of patients, we identified the dynamics of clinical indicators (Tables 6, 7).
Table 6 Dynamics of pain syndrome in observation groups
| 1st group | 2nd group | 3rd group | 4th group | |||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Pain in bed at night | 41,5±3,82 | 40,9±4,11 | 41,2±2,63 | 35,1±2,41* | 40,6±3,15 | 32,4±2,77* | 41,2±3,87 | 15,3±1,96** |
| Pain while sitting and lying down | 43,2±4,03 | 42,9±4,14 | 44,3±3,84 | 41,6±3,50* | 42,3±2,90 | 38,2±3,33* | 43,8±3,97 | 16,5±1,85** |
| Pain in an upright position | 45,4±4,12 | 42,3±3,96 | 46,2±3,76 | 38,3±2,97* | 45,7±3,42 | 41,5±3,64* | 45,8±3,52 | 17,1±1,93** |
| Pain with active movements of the upper limbs | 46,3±4,67 | 44,7±4,43 | 47,1±4,85 | 42,6±3,92* | 46,4±4,71 | 42,1±4,25* | 46,7±4,72 | 18,9±2,03** |
| Pain with passive movements of the upper limbs | 49,7±5,11 | 46,2±4,93 | 50,2±4,98 | 43,9±2,86* | 49,8±5,09 | 41,6±4,11* | 50,1±5,13 | 20,1±4,35** |
| Pain on palpation | 53,9±4,96 | 49,4±3,85 | 54,6±4,87 | 45,2±3,76* | 54,2±4,93 | 46,1±3,86* | 54,4±4,63 | 22,6±3,72** |
Note: * p<0.05; ** — p<0.01 – significance of intergroup differences after treatment.
Table 7 Dynamics of the severity of parameters of the WOMAC questionnaire in observation groups
| 1st group | 2nd group | 3rd group | 4th group | |||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Section A. Pain | 42,6±3,57 | 39,5±3,26 | 43,5±4,01 | 19,6±2,82** | 44,8±4,16 | 21,4±3,18** | 44,5±3,94 | 11,2±1,37** |
| Section B. Stiffness | 25,4±3,25 | 22,1±2,97 | 26,7±3,84 | 19,1±1,74* | 28,3±4,06 | 18,2±2,45* | 27,9±4,11 | 13,5±2,06** |
| Section B. Difficulties in daily activities | 52,5±4,62 | 45,3±4,39 | 54,1±4,48 | 23,5±3,26** | 55,3±4,69 | 24,4±2,83** | 53,6±4,37 | 18,7±2,12** |
Note: * p<0.05;
** — p<0.01 – significance of intergroup differences after treatment. As a result of the therapy, both in the second and third groups, and during combination therapy, there was a positive dynamics of pain, stiffness and difficulties in daily activities, which proves the high effectiveness of monotherapy with the drugs “Artradol” and “Artracam”, with the synergistic effect of their joint administration .
Table 8 Dynamics of quantitative assessment of the coordination test “Get up and go”
| 1st group | 2nd group | 3rd group | 4th group | |||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Inability to get up from a chair without support (sec) | 9,1±3,75 | 8,6±2,65 | 8,9±4,26 | 4,5±2,54* | 8,1±3,51 | 3,6±2,57* | 9,0±4,05 | 0,6±0,41** |
| Inability to get up from a chair without support (CHS points) | 9,2±3,84 | 8,5±2,74 | 8,5±3,97 | 4,4±2,15* | 8,0±3,38 | 3,5±2,62* | 8,8±3,63 | 0,5±0,34** |
Note: * p<0.05;
** — p<0.01 – significance of intergroup differences after treatment. Significant dynamics of the coordination test “Get up and go” characterizes the expansion of daily activities and improvement of self-care of the observed patients.
The study assessed the effectiveness of therapy by the doctor and the patient, which was calculated against the background of continuous use of NSAIDs.
Table 9 Efficacy of initial therapy in patients included in the study, assessed by physicians and patients
| Grade | Number of patients | ||
| n | % | ||
| sick | Very good | 0 | 0 |
| Fine | 19 | 1583 | |
| Satisfactorily | 61 | 5084 | |
| Badly | 38 | 3166 | |
| Very bad | 2 | 167 | |
| Doctor | Very good | 0 | 0 |
| Fine | 26 | 2167 | |
| Satisfactorily | 90 | 75 | |
| Badly | 4 | 333 | |
| Very bad | 0 | 0 | |
Table 10 Adverse side effects identified during observation in the study groups
| Undesirable side reactions | Group 1 (n=30) | Group 2 (n=30) | Group 3 (n=30) | Group 4 (n=30) | ||||
| Gastralgia | 1 | 333% | 2 | 667% | 1 | 333% | 2 | 667% |
| Diarrhea | 1 | 333% | 1 | 333% | 1 | 333% | 1 | 333% |
| Flatulence | 1 | 333% | 0 | 0% | 0 | 0% | 0 | 0% |
| Facial redness | 1 | 333% | 0 | 0% | 0 | 0% | 0 | 0% |
| Synovitis | 0 | 0% | 0 | 0% | 1 | 333% | 1 | 333% |
According to the data presented in Table 10, the drugs “Artradol” and “Artracam” are well tolerated, both in monotherapy and in combination therapy. The occurrence of undesirable side reactions was observed during monotherapy with Artradol in 10%, and with Artracam in 9.99%. During a course of combination therapy, an increase in undesirable side reactions was revealed to 3.32%. No serious adverse events were identified.
The association of adverse events with drug intake was possible or probable. To correct undesirable side effects, dietary measures were carried out; if gastralgia developed, NSAIDs were discontinued and antispasmodics were prescribed (once in all groups). When nausea occurred, the duration of which did not exceed 10 hours, metoclopramide was prescribed. The appearance of flatulence and facial redness did not require medication. Synovitis was controlled by prescribing NSAIDs in adequate doses. There was no need to discontinue therapy in the observation groups.
The need for NSAIDs in the observation groups decreased: after two weeks in 24 (80%) patients in group 2, in 23 (76.7%) in group 3, in 27 (90%) in group 4. After 1 month, the need for regular NSAID use continued to decrease. 27 (90%) in group 2 refused to take non-steroidal drugs, 28 (93.3%) in group 3, and 29 (96.7%) in group 4.
While monitoring patients with comorbid pathology, we did not identify any negative drug interaction reactions, which did not require changing the recommendations of specialists in the relevant field.
Discussion
News of medicine and pharmacology in the field of treatment of diseases of the joints and spine
Artradol lyophilisate for the preparation of solution for intramuscular administration 100 mg, 10 ampoules
Registration Certificate Holder
INKAMFARM (Russia)
Dosage form
Medicine - Artradol® (Artradol)
Description
Lyophilisate for preparing a solution for intramuscular administration
white or white with a yellowish tint porous mass compacted into a tablet.
1 amp.
chondroitin sulfate sodium 100 mg
Ampoules with a capacity of 2 ml (5) - plastic trays (2) - cardboard packs. Ampoules with a capacity of 2 ml (10) - cardboard holders (1) - cardboard packs.
Indications
Degenerative-dystrophic diseases of the joints and spine (osteoarthrosis of peripheral joints, intervertebral osteoarthrosis and osteochondrosis), to accelerate the formation of callus in fractures.
Contraindications for use
Hypersensitivity to chondroitin sulfate, tendency to bleeding, thrombophlebitis; for intramuscular administration and oral administration - children under 18 years of age, pregnancy, breastfeeding; for intra-articular injection - the presence of active inflammatory and infectious processes in the joint, the presence of active skin disease and skin infection in the area of the intended injection.
Carefully
Simultaneous use with direct anticoagulants.
pharmachologic effect
The agent that affects phosphorus-calcium metabolism in cartilage tissue is a high-molecular mucopolysaccharide. It has chondrostimulating, regenerating, anti-inflammatory and analgesic effects. Chondroitin sulfate is involved in the construction of the main substance of cartilage and bone tissue. It has chondroprotective properties, enhances metabolic processes in hyaline and fibrous cartilage, subchondral bone; inhibits enzymes that cause degradation (destruction) of articular cartilage; stimulates the production of proteoglycans by chondrocytes. Helps reduce the release of inflammatory mediators and pain factors into the synovial fluid, suppresses the secretion of leukotrienes and prostaglandins. Slows down bone resorption and reduces calcium loss, accelerates bone tissue restoration processes. Chondroitin sulfate slows the progression of osteoarthritis and osteochondrosis. Promotes the restoration of the joint capsule and cartilaginous surfaces of the joints, prevents the collapse of connective tissue, and normalizes the production of joint fluid.
Possessing structural similarity to heparin, it can potentially prevent the formation of fibrin thrombi in the synovial and subchondral microvasculature.
Drug interactions
The effect of indirect anticoagulants, antiplatelet agents, and fibrinolytics may be enhanced, which requires more frequent monitoring of blood coagulation parameters when used together.
Dosage regimen
Orally for adults - 1.5-1 g 2 times a day.
IM - 100 mg chondroitin sodium sulfate every other day. If well tolerated, the dose is increased to 200 mg, starting with the fourth injection. The course of treatment is 25-30 injections. If necessary, repeated courses of treatment are possible after 6 months.
For intra-articular administration, a single dose is 200 mg. Frequency and duration of use - according to a special scheme.
Side effect
Allergic reactions: uncommon - skin itching, erythema, urticaria, dermatitis, angioedema.
From the digestive system: often - diarrhea, abdominal pain, nausea; frequency unknown - vomiting.
Local reactions: pain and hemorrhage at the injection site.
special instructions
In case of allergic reactions or hemorrhages, treatment should be discontinued.
Use during pregnancy and breastfeeding
Restrictions during pregnancy - Contraindicated. Restrictions when breastfeeding - Contraindicated. Use during pregnancy and breastfeeding is contraindicated.
Use in elderly patients
Restrictions for elderly patients - With caution. There are no special instructions for use in elderly patients.
Use in children
Restrictions for children - Contraindicated. Use in children and adolescents under 18 years of age is contraindicated.