Introduction
Anemia is a global problem: approximately 25–30% of people suffer from this disease, and half of all anemia is caused by iron deficiency [1, 2].
Diagnostic criteria for anemia, according to WHO, are hemoglobin levels below 130 g/l in men of all ages and postmenopausal women, for non-pregnant women of reproductive age less than 120 g/l, for pregnant women - less than 110 g/l. These WHO standards are used by physicians in most countries for professional consistency purposes [3, 4]. According to the degree of severity, according to the level of hemoglobin (Hb), anemia is divided into mild (Hb≥90 g/l), moderate (Hb 90–70 g/l), severe (Hb 69–50 g/l) and extremely severe anemia (<50 g/l) [5, 6].
Iron deficiency anemia (IDA) is an acquired disease that is characterized by a reduced iron content in the blood serum, tissue depots, and bone marrow, resulting in the development of hypochromia and trophic disorders in tissues [7–9]. IDA is one of the most common pathological conditions in the world, and in women of childbearing age it is in first place in terms of occurrence [7].
Add foods rich in vitamin B12 and iron to your diet
Vitamin B12 is found in meat (mostly red), liver, fish and seafood (there is a lot of it in oysters). There are also plant sources of vitamin B12: tofu, lentils, raisins, oatmeal and cereal flakes, but the risk of developing anemia in vegetarians is still quite high. However, there is a way out - vitamin B12 can be taken in the form of nutritional supplements, for example, as part of a vitamin complex.
Good sources of iron: dark green leaves (spinach, cauliflower, Brussels sprouts, broccoli). The leaders in iron content are parsley and artichokes. Sweet sources are dried apricots and prunes.
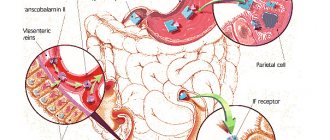
Iron metabolism
It is known that iron is an important trace element, which is used mainly as a component of heme in erythrocytes for oxygen transport, and is present in smaller quantities in muscles in the form of heme-myoglobin and in the liver in the form of ferritin [10]. Its sufficient level is necessary to maintain physiological homeostasis [11]. However, excess iron levels can lead to cell death through the formation of free radicals and lipid peroxidation of biological membranes, toxic damage to proteins and nucleic acids. It is important that both iron deficiency and overload can have catastrophic consequences for the body, therefore the content of this microelement is strictly regulated [7, 9, 12–14].
The body of a healthy person contains about 3–5 g of iron, most of which, 2100 mg, is found in blood cells and bone marrow. Approximately 2.5 g of this iron is present in hemoglobin for oxygen transport, and another 2 g is stored as ferritin, mainly in the bone marrow, liver and spleen [9]. In the bone marrow, iron is used to form hemoglobin, liver iron is the main reserve of the microelement, and reticuloendothelial cells of the spleen remove old red blood cells. Finally, relatively small amounts of iron (approximately 400 mg) are present in cellular proteins such as myoglobin and cytochromes, and approximately 3–4 mg is bound to transferrin in the circulation [10, 15]. Almost all metabolically active iron is bound to proteins, and free iron ions can be present in extremely low concentrations.
Under natural conditions, no more than 0.05% (<2.5 mg) of the total amount of iron is lost daily due to exfoliating epithelium of the skin and gastrointestinal tract (GIT), as a result of sweating [12, 13, 16, 17]. The processes of absorption, recycling and storage of iron reserves are regulated by a special hormone - hepcidin, which is produced by liver cells. Under physiological conditions, hepcidin production is controlled by a complex interaction of signals, primarily the level of iron in the blood and the degree of oxygenation of liver tissue. Under pathological conditions, its production is regulated by proinflammatory cytokines, of which interleukin-6 plays a major role [7, 12, 13, 18].
Iron deficiency is detected in people of all age and social groups, but more often in women of reproductive age, young children and the elderly [5, 6]. The main reasons for the development of iron deficiency include decreased food intake, decreased absorption and blood loss. In developed, resource-rich countries, adult diets are almost always adequate, and the most common cause of iron deficiency is blood loss [2, 6].
Where to look for iron
Iron, contrary to popular belief, is far from a rare trace element. Many foods are rich in it - buckwheat, pomegranates, legumes, parsley, peas, prunes, dried apricots and so on. The problem is that only divalent iron, which is present in meat products, is absorbed from food. Plant foods contain ferric iron, which must be converted into divalent iron in the body. But this process does not always go smoothly - with low stomach acidity, for example, iron from plant products is not retained in the body at all.
The best way to level out your hemoglobin levels is to become a meat eater. It is also important to eat meat correctly - along with foods rich in vitamin C (fresh vegetables, fruits, herbs), which increases the absorption of iron.
Click to enlarge
If the “iron” diet does not help, and other causes that could cause persistent anemia are excluded, doctors prescribe iron supplements. You will have to take them for a long time, until all indicators normalize. You cannot stop taking it as soon as signs of improvement appear. And of course, the type of drug, dose and method of administration must be prescribed by a doctor.
While taking medications, you will have to review your diet. Tannins found in tea and coffee and calcium found in dairy products impair iron absorption, as do antacids (against heartburn). Therefore, they cannot be taken simultaneously with “iron” drugs.
Article on the topic
Lose your cool. Why does a person feel cold all the time?
Prevalence of IDA
Iron deficiency anemia in women is a common pathological condition. Thus, according to WHO (2015), severe iron deficiency is observed in every third woman of reproductive age and in every second pregnant woman, being an important cause of chronic fatigue and poor health, and the third most common cause of temporary disability in women aged 15– 44 years old [12, 19]. In the Russian Federation, despite active preventive and therapeutic measures, the prevalence of IDA remains very high. For example, in Moscow, anemia occurs in almost 38% of gynecological patients [20, 21] and is the most common concomitant pathological process and the first manifestation of the underlying disease, determining the severity of its course and treatment tactics. The main reasons for the development of IDA in women are heavy menstrual bleeding, pregnancy, childbirth (especially repeated ones) and lactation. Anemia often accompanies uterine fibroids, adenomyosis, hyperplastic processes in the endometrium, and ovarian dysfunction. During normal menstruation, 30–40 ml of blood is lost (which is equivalent to 15–20 mg of iron). The critical level corresponds to a blood loss of 40–60 ml, and with a blood loss of more than 60 ml, iron deficiency develops. In women suffering from abnormal uterine bleeding of various origins, the amount of blood lost during one menstruation can reach 200 ml (100 mg of iron) or more. In such situations, the loss of iron exceeds its intake and IDA gradually forms [20].
The most important medical and social problem is anemia in pregnant women, which, according to WHO, is detected in 24–30% of women in economically developed countries and in more than 50% of women in countries with low economic levels [3, 22].
A survey of pregnant women conducted as part of clinical studies in the 2000s showed a high incidence of anemia even among residents of prosperous European countries. Thus, in Belgium (n=1311), Switzerland (n=381) and Germany (n=378) iron deficiency was diagnosed in 6% and 23% (serum ferritin (SF) <15 μg/l) in the first and third trimesters, respectively, of Belgian women; in 19% (SF<12 µg/l) - in Switzerland and Germany. The prevalence of IDA (Hb <110 g/L, SF <15 μg/L) was 16% in Belgium and 3% in Switzerland, although 65–66% of Belgian and Swiss women received dietary iron supplementation during pregnancy. In Germany, IDA was diagnosed in 12% of women [23].
In Russia, according to the Ministry of Health, the incidence of anemia in pregnant women varies from 39% to 44%, in postpartum women - from 24% to 27% [24]. A 2021 systematic review and meta-analysis found that in low- and middle-income countries, pregnancy anemia increases the likelihood of preterm birth by 63%, low birth weight by 31%, perinatal mortality by 51%, and neonatal loss by 2 ,7 times [25].
During pregnancy, there is a significant physiological increase in the need for iron for the normal functioning of the placenta and fetal growth. The total amount of iron required for a normal pregnancy is 1000–1200 mg. To complete a normal pregnancy without developing iron deficiency, a woman must have iron stores in the body at conception of ≥500 mg, which corresponds to an SF concentration of 70–80 μg/L [20, 23].
Clinical manifestations of iron deficiency
The clinical manifestations of iron deficiency are diverse and can be reduced to two main syndromes: hypoxic and sideropenic. Hypoxic syndrome combines symptoms common to all anemia: pallor, increased heartbeat, tinnitus, headache, weakness. Sideropenic syndrome includes taste perversion, dry skin, changes in nails, hair loss, angular stomatitis, burning tongue, and dyspepsia. The variety of clinical symptoms of iron deficiency can be explained by a wide range of metabolic disorders, which are caused by dysfunction of iron-containing and iron-dependent enzymes [6, 8, 9, 26, 27].
Symptoms that are less associated with anemia, but may be a manifestation of iron deficiency, include neurotic reactions and neurasthenia, decreased performance, muscle weakness and general tolerance to physical activity, disorders of metabolic processes in the myocardium, peripheral circulation and microcirculation, low-grade fever [6] . Exotic symptoms of IDA include urgency to urinate/defecate, urinary incontinence due to weakening of the sphincter apparatus, and difficulty swallowing due to atrophic changes in the esophageal mucosa [5, 12]. According to modern data, restless legs syndrome (Willis-Ekbom disease) may be one of the most common clinical manifestations of iron deficiency [28, 29].
Anti-infective immunity disorders in patients with impaired micronutrient status and IDA are complex [30]. On the one hand, iron deficiency prevents the development of pathogenic microorganisms that require iron for their own growth and reproduction. On the other hand, iron deficiency indirectly leads to disruption of cellular resistance mechanisms and to infections (decreased microbicidal activity of granulocytes, impaired proliferation of lymphocytes). In general, the predisposition of patients with IDA to the development of infectious diseases is not as great as previously thought. Moreover, treatment of IDA with parenteral iron supplements increases the risk of developing infections, probably due to the availability of administered iron for the rapid growth of pathogenic microorganisms [7, 26].
Symptoms of anemia
Lack of hemoglobin leads to oxygen starvation, manifested by symptoms typical of anemia such as:
General weakness
With anemia, muscle tissue does not receive sufficient nutrition. The patient feels constantly tired, he does not have enough strength for normal life activities.
Drowsiness
The body lacks strength, which means it needs additional rest. Drowsiness develops. A person suffering from anemia is almost never alert; as a rule, he wants to sleep.
Pallor
Pale skin due to anemia is caused by a decrease in the number of red blood cells (erythrocytes) in the blood.
Dizziness
With anemia, the brain also receives insufficient nutrition. This may cause dizziness.
flickering
The flickering of “flies” before the eyes is caused by insufficient nutrition of the structures of the visual apparatus.
Fainting
If the decrease in hemoglobin is significant, fainting is possible - episodes of loss of consciousness.
Headache
With anemia, headaches are often observed.
More about the symptom
Cardiopalmus
You may experience palpitations with little physical activity or at rest.
More about the symptom
Dyspnea
Shortness of breath with anemia is caused by the fact that the body tries to compensate for the lack of oxygen by increasing its supply. Breathing becomes more frequent.
More about the symptom
Diagnosis of IDA
Diagnosis of IDA is based on the characteristic clinical and hematological picture of the disease and the presence of laboratory evidence of absolute iron deficiency.
When performing a physical examination of patients with suspected IDA, it is necessary to pay attention to the characteristic signs of sideropenic and hypoxic syndromes given earlier. However, the symptoms of anemia and sideropenia have low diagnostic value and do not allow a reliable diagnosis of IDA to be established. Laboratory tests are of decisive importance in the diagnosis of IDA.
First of all, in patients with anemic syndrome, a general (clinical) blood test is performed with an assessment of hematocrit, the level of erythrocytes, reticulocytes, the average content and average concentration of hemoglobin in erythrocytes and the size of erythrocytes [17]. With IDA, there is a decrease in the level of hemoglobin, hematocrit, the average content and average concentration of hemoglobin in erythrocytes, and the average volume of erythrocytes. The red blood cell count is usually within normal limits. Reticulocytosis is uncommon but may be present in patients with bleeding. Typical morphological signs of IDA are hypochromia of erythrocytes and anisocytosis with a tendency to microcytosis [5, 6, 31, 32].
However, the listed morphological characteristics do not allow distinguishing IDA from the so-called anemia of chronic diseases, which is based on redistribution of iron deficiency associated with the presence of a focus of inflammation, infection or tumor in the body. Therefore, all patients with suspected IDA need to examine serum indicators of iron metabolism - ferritin, transferrin and iron levels, total serum iron binding capacity (TIBC), and also determine the calculated indicator - transferrin iron saturation coefficient (TIS) [6, 7, 19, 32 ].
Distinctive signs of true IDA are a low level of SF, reflecting the depletion of tissue iron reserves, and increased levels of PVSS and transferrin. Serum iron levels and the IF coefficient are in typical cases reduced, but the presence of normal and even elevated values does not exclude the diagnosis of IDA, since taking iron-containing drugs on the eve of the study, a meat diet, or a previous (10–14 days before) red blood cell transfusion can greatly distort the serum iron level and, accordingly, the NTG coefficient, which must be taken into account when assessing the results of the study [7, 9, 26].
The development of IDA is preceded by a period of latent iron deficiency, the laboratory criteria of which are low levels of serum iron and ferritin against the background of normal hemoglobin levels.
The study of serum indicators of iron metabolism must be combined with basic studies, which include: general urine analysis, biochemical blood test (total protein, albumin, total bilirubin, direct bilirubin, AST, ALT, creatinine, urea, alkaline phosphatase, γ-glutamine transpeptidase) with determining the main indicators of the functional state of the liver, kidneys, pancreas, as well as screening for viral hepatitis B and C, HIV infection, syphilis. Carrying out these studies is necessary for the correct interpretation of serum indicators of iron metabolism, since the state of iron metabolism, on the one hand, is an “endocrine function of the liver”, on the other hand, it changes significantly in the presence of inflammatory, destructive or tumor processes in the liver and other vital organs [ 7, 9, 26].
It is important to note that microcytic hypochromic anemia is a characteristic morphological sign of β-thalassemia, severe forms of which are associated with profound anemia and pronounced signs of iron overload (increased levels of serum ferritin and LTFA, decreased levels of transferrin and TBL). However, mild subclinical forms of thalassemia, occurring with mild microcytic hypochromic anemia, are often regarded as iron deficiency without studying serum indicators of iron metabolism, which entails the prescription of inadequate ferrotherapy, which can lead to the accelerated development of tissue iron overload. In this regard, IDA must be differentiated from anemias occurring with iron overload: α- and β-thalassemia, porphyria, lead intoxication [9, 32].
Treatment of IDA
The goal of treatment for IDA is to replenish iron stores in the amount necessary to normalize hemoglobin levels (in women 120–140 g/l) and replenish tissue iron stores (SF>40–60 μg/l). For treatment and prevention, oral preparations of iron salts are used, most often ferrous sulfate; in recent years, iron fumarate, iron gluconate, or combination preparations have also been actively used. The quantitative and qualitative composition of iron medicinal preparations varies greatly; depending on this, the preparations are divided into high- and low-dose, single-component and combined. In accordance with the WHO recommendation, the optimal dose of iron for the treatment of IDA is 120 mg/day, for the prevention of iron deficiency - 60 mg/day [7]. Approximately 20% of patients develop diarrhea or constipation during treatment, which can be relieved with symptomatic therapy. Signs of stomach irritation, such as nausea and epigastric discomfort, are minimized by taking iron supplements with meals or reducing their dose. The use of high-dose iron supplements is accompanied by an increase in the frequency of side effects from the gastrointestinal tract. The duration of treatment is determined by the depth of the initial iron deficiency and can vary from 1 month. up to 3 months [6–9, 19].
There is now accumulating evidence that low-dose iron supplements, given in short courses (2 weeks per month) or in an alternative regimen (every other day for a month), have higher effectiveness and lower incidence of side effects than previously used high-dose preparations. including in the form of repeated (2–3 times a day) doses [7, 33, 34].
It is important to emphasize that high doses of iron supplements may be associated with oxidative cytotoxic effects of unabsorbed iron on the intestinal mucosa, which is clinically manifested by side effects such as nausea, vomiting, constipation or diarrhea. Other adverse effects of unabsorbed iron include disturbances in the composition of the gut microbiome, with a decrease in lactobacilli and bifidobacteria and an increase in potential pathogens ( Enterobacteriaceae
), which entails the development of inflammation and diarrhea [31]. Modification of the iron supplementation regimen, such as switching from daily to alternative and from 2–3 times to once a day, may increase the effectiveness of treatment and improve its tolerability [33–35].
An example of a modern iron drug is the combination drug Ferretab® comp. One capsule of the drug includes 3 mini-tablets containing iron fumarate 163.56 mg (equivalent to 50 mg of iron), 1 mini-tablet of folic acid 0.54 mg (equivalent to 0.5 mg of dry matter) and auxiliary components. The folic acid mini-tablet dissolves within minutes and is rapidly absorbed in the jejunum. Iron is built into the inert matrix of the mini-tablet, which avoids high concentrations upon release and prevents irritation of the mucous membrane. Iron absorption occurs directly in the duodenum and upper jejunum. During the passage of the mini-tablet through the intestines, continuous release and absorption of iron occurs, providing a prolonged effect of the drug with a single daily dose.
The advantage of the drug is its combined composition: active divalent iron (iron fumarate) and folic acid, which is a hematopoietic cofactor vitamin necessary for the growth and differentiation of bone marrow erythroid cells. This increases the effectiveness of treatment, as demonstrated in a meta-analysis in 2015 [36]. During pregnancy, folic acid protects the fetus from the effects of teratogenic factors [37].
The drug does not have a specific taste or smell of iron, does not stain tooth enamel, is well tolerated and convenient for use: 1 tablet per day.
Treatment with parenteral iron is recommended for IDA in cases of ineffectiveness, poor tolerability, or contraindications to the use of oral iron medications [4, 6, 7, 9]. The use of intravenous iron supplements is indicated primarily for absorption disorders due to previous extensive intestinal resection, for inflammatory bowel diseases (ulcerative colitis, Crohn's disease) and malabsorption syndrome, for chronic kidney disease in the predialysis and dialysis periods, and also if necessary to obtain a quick effect in in the form of replenishing iron reserves and increasing the efficiency of erythropoiesis (for example, before major surgical interventions) [6, 7, 9, 27].
Intravenous infusions of iron preparations are associated with the danger of anaphylactic shock (in 1% of cases), the development of iron overload and toxic reactions associated with the activation of free radical reactions of biological oxidation (lipid peroxidation) by iron ions.
Intramuscular administration of iron preparations has not been used for a long time due to low efficiency, the development of local hemosiderosis and the risk of developing infiltrates, abscesses and even myosarcoma at the injection site.