What to do if you have high stomach acidity?
Stomach problems are one of the most common ailments that plague a huge number of people. Sometimes it is severe pain, sometimes constant nausea, and often vomiting and lack of appetite. There are many reasons for the occurrence of such problems, but one of the main reasons why a person feels discomfort is increased stomach acidity.
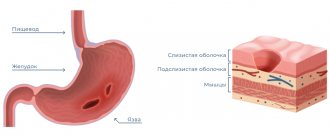
The natural physiological increase in acidity that occurs after eating occurs relatively unnoticed in a healthy person. It is also considered normal if the pH temporarily increases after eating certain foods - citrus fruits, tomatoes and tomato juice (paste, sauce), alcoholic drinks, etc. A condition in which the level of hydrochloric acid significantly exceeds the norm, regardless of food intake, is considered pathological.
Causes
The most common cause of increased stomach acidity is a nutritional factor, i.e., improper, irrational nutrition. Spicy, salty, fatty foods, and alcoholic drinks have an irritating effect on the gastric mucosa, as a result of which the parietal cells begin to secrete hydrochloric acid in greater quantities than required.
The nutritional factor also includes too rapid absorption of food. In this case, a poorly chewed bolus of food enters the stomach, not sufficiently moistened with saliva, containing too large particles. To digest it, a larger amount of gastric juice is required, and therefore hydrochloric acid, which leads to increased acid production, and therefore to an increase in the acidity of gastric juice.
Other causes of increased stomach acidity may include:
- Infection with the bacterium Helicobacter pylori. This is a unique microorganism that can survive in an acidic environment. Once in the stomach, bacteria produce urease, which has an irritating effect on its walls. In an effort to destroy these bacteria, stomach cells intensively synthesize hydrochloric acid and pepsin.
- Chronic stress. In itself, it does not have a negative effect on the state of the digestive system, however, being in a depressed state, a person stops eating properly, often smokes, drinks alcohol, which negatively affects the gastric mucosa.
- Long-term use of non-steroidal anti-inflammatory drugs and/or corticosteroids, as they have an irritating effect on the gastric mucosa.
- Smoking. Nicotine has a stimulating effect on parietal cells, resulting in an increase in stomach acidity.
Signs of low acidity
- the skin became dry and flaky;
- acne appears on the face;
- bloating appears after eating;
- Breath smells like “rotten” eggs.
The described symptoms in combination with a patient interview are enough to correctly determine the acidity of the stomach.
Consequences of low acidity
Allergy
Due to a lack of acid, food is poorly digested and its particles enter the bloodstream. The body reacts to them as if they were poisonous elements and begins to intensively produce antibodies.
Helminthiasis
A lack of acid creates ideal conditions for the colonization of the intestinal tract by various bacteria and worms.
Stool disorders
The lack of an alkaline environment interferes with the normal digestion of foods, which is why undigested pieces of food decompose inside the intestines. Rotting stimulates the proliferation of pathogenic bacteria, which produce gases - bloating appears, constipation or diarrhea develops.
Depressive states
A healthy nervous system is the key to strong nerves and good mood. If nerve impulses are poorly transmitted between cells, a person becomes apathetic, depressed, and cognitive abilities are impaired: reactions slow down, memory deteriorates, and concentration decreases. The rapid transmission of impulses is ensured by amino acids, which are formed as a result of the breakdown of proteins. The low acidity of gastric juice does not allow proteins to undergo the entire breakdown process.
Constipation and diarrhea
In an “alkalinized” body, food takes a long time to digest, its remains decompose, provoking attacks of flatulence, constipation and diarrhea.
Depression
Due to a lack of acids, proteins cannot be fully broken down into amino acids. Their deficiency leads to a disruption in the transmission of impulses between nerve cells, which leads to apathy and depression, as well as a deterioration in the cognitive functions of the brain (reaction, memory, etc.).
Symptoms
Increased acidity very often occurs against the background of an imbalance in the process of regulating the acidity of the juice, as well as its neutralization. That is why, when acidity increases, a person may experience the following symptoms:
- Bloating.
- State of apathy, bad mood.
- Heartburn. It usually occurs either immediately after eating, or at the moment when a person takes a lying position. Heartburn is the reflux of stomach acid into the esophagus. The juice irritates the mucous membrane and therefore a strong burning sensation occurs, which is localized mainly in the Adam's apple area.
- Feeling of heaviness. This usually happens even after a small meal or snack.
- Pain. It can be either strong or not very strong. It occurs at the moment when acid enters the duodenum, or rather into its lumen. In addition, another cause of pain will be acid getting into damaged mucous areas. At the moment when their interaction occurs, the person feels a strong pain attack, which can last for several minutes. At the same time, it is difficult for a person to even move, the pain is so severe.
- Belching, which usually tastes either sour or bitter. Moreover, it appears mainly after eating.
- Problems with appetite. Due to the fact that after each meal a person suffers from pain or heartburn, appetite gradually decreases.
- Problems with stool. In some people this may be diarrhea, which is very difficult to relieve even with medications, and in some people it may be constipation for a long time.
- A feeling of constant discomfort in the stomach area, which can be manifested by stretching. Heaviness, tingling, etc.
And most importantly, not a single person has yet managed to independently relieve the symptoms of high stomach acidity. Of course, there are several medications that can help temporarily alleviate the condition. But only a specialist can find out the true cause of the increase in acidity and prescribe treatment.
Popular questions about hypoacid gastritis
How to determine low stomach acidity?
Only pH-metry can give an accurate determination of acidity. But you can suspect its low level if you can safely eat sour lemon.
How to distinguish gastritis with high and low acidity?
There are certain signs that characterize different types of acidity. If it is heartburn or sour belching, then most likely this indicates increased acidity. Low blood pressure is characterized by belching with the smell of rotten eggs, which indicates poorly digested food.
What should you not eat if you have gastritis with low acidity?
If such gastritis is detected, it is better to forget about smoked, fatty, spicy foods, fermented cheeses, chocolate, coffee and carbonated drinks.
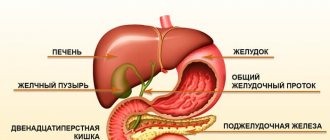
Diagnostics
The degree of acidity can be accurately determined only on an outpatient basis. For this purpose, the following gastroenterological studies are used:
- gastroduodenoscopy - endoscopic examination using a probe;
- daily pH-metry - a procedure carried out using acidogastrometers for 24 hours;
- assessment of the acidity level by the degree of staining of urine with ion exchange resins;
- fractional sounding of the digestive organ, during which a special tube sucks out the contents in different parts of the stomach with further laboratory analysis.
In modern practice, the method of daily pH-metry is recognized as the most accurate, since it allows you to measure the acidity of the stomach directly in its cavity, which allows you to minimize distortion of the analysis results.
How to diagnose hypoacid gastritis
If you notice similar symptoms, you should consult a specialist in the field of gastroenterology for diagnosis. To determine the clinical picture and make a correct and accurate diagnosis, the doctor usually conducts an examination, finds out complaints and prescribes tests and studies, as a result of which the type of pathology and how affected the gastric mucosa is determined. To determine chronic hypoacid gastritis use:
- visual examination of the stomach using a gastroscope (gastroscopy);
- endoscopic biopsy (taking material for biopsy);
- pH-metry of the acidity of secreted gastric juice;
- blood tests and coprogram.
Having collected a complete picture as a result of instrumental and laboratory studies, the doctor proceeds to drawing up a treatment program.
Consequences
Due to the excess hydrochloric acid content in the stomach and the resulting indigestion, the patient may experience a sharp loss of body weight. A deficiency of nutrients, vitamins and minerals leads to brittle hair and nails, deterioration in the appearance of the skin - it becomes gray, dull, dehydrated, loses tone and becomes vulnerable to mites. Teeth are also subject to negative effects.
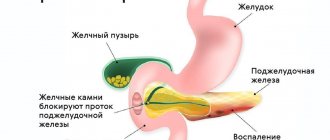
A more serious complication of increased gastric pH is the occurrence of gastritis and peptic ulcers. However, the development of these diseases most often requires the presence of additional negative factors (Helicobacter pylori infection, unhealthy lifestyle, unhealthy diet).
Opportunities for quickly eliminating symptoms and normalizing the level of stomach acidity allow us to make favorable prognoses for patients who are attentive to their health and follow the doctor’s recommendations.
Diagnostic examination methods
Determination of the acidity of digestive juice is carried out in specialized laboratories. The treating gastroenterologist will be asked to undergo the following diagnostic procedures:
- Intragastric pH-metry, which allows you to determine the level of hydrochloric acid in certain areas of the digestive tract.
- Fractional sounding performed on an empty stomach will establish the physiological characteristics of the secretory function of the stomach.
- Intragastric pH-metry is considered the most informative method of diagnostic research, which allows you to accurately determine the level of acidity inside the stomach.
In addition, instrumental examination includes ultrasound of the abdominal cavity, gastroduodenoscopy and contrast X-ray scanning.
See also: How to determine stomach acidity at home?
How to reduce stomach acidity?
Treatment for high acid levels involves the use of the following medications:
- Proton pump blockers. These include Omeprazole and Lansoprazole.
- Histamine blockers. Doctors often prescribe Ranitidine and Famotidine. They normalize digestion and reduce the amount of acid.
- Antiacid drugs. The most effective are Almagel and Maalox. They are prescribed for inflammation of the stomach and to normalize the level of hydrochloric acid secretion.
- To eliminate stomach pain, experts prescribe Motilium or Domidon.
A well-known remedy for neutralizing hydrochloric acid is baking soda. However, experts recommend not to abuse it during treatment.
First aid for hyperacidity
Antacids help neutralize the aggressive effect of hydrochloric acid, which is the main component of gastric juice. Simple soda has a similar effect. Soda does not have a cumulative effect; it has a one-time effect, neutralizing the acid present in the stomach. However, the powder contains sodium, which may have a negative effect on the course of some cardiovascular diseases.
Antacid medications contain magnesium, aluminum, bismuth and other components that have additional effects:
- antiseptic;
- antibacterial;
- regenerating.
Antacids are available in the form of a thick gel and in tablet form. Medicines envelop the mucous walls of the digestive tract, creating a specific barrier that protects the membranes from the pathogenically aggressive effects of hydrochloric acid. Preparations:
- Phosphalugel;
- Almagel;
- Rennie;
- Gastal.
Drug treatment
The possibilities of modern medicine in treating high acidity of the stomach are quite large. There are a huge number of drugs with different mechanisms for suppressing the acid-forming function of parietal cells. But you need to be able to find a competent approach to their differentiated use. The basics for the correct use of such funds are outlined below.
- Pantoprazole, omeprazole, lansoprazole, razol, Proxium, Nexium, etc. Today, they are rightfully considered the main antisecretory drugs. Most effectively and selectively block the production of hydrochloric acid. The only drawback of drugs in this group is that they cannot be used in pediatric practice.
- Maalox, Almagel, Phosphalugel, Venter, Gastal. They have a combined effect on the mucous membrane: neutralizing acid and protecting the inflamed mucous membrane from irritation due to the formation of a barrier film. The drugs do not affect the level of secretory activity of acid-forming cells. Can be used as a basic remedy for hyperacid gastritis.
- Rennie, Gaviscon. They have a quick healing effect. Prescribed to relieve periodic attacks of heartburn. They cannot be used as a basic treatment, although they do not cause rebound syndrome. They can be classified as drugs of purely symptomatic therapy.
- Ranitidine, quamatel (famotidine). Effectively reduce the level of general gastric secretion, including hydrochloric acid. They have a symptomatic and pathogenetic effect, stopping not only painful manifestations in the form of heartburn, but also influencing the root causes of its occurrence.
- Vikalin, vis-nol, de-nol, vikair, gastro-norm. They are used as drugs of choice for high acidity, accompanied by the formation of ulcers of the mucous membrane of the gastroduodenal complex associated with Helicobacter pylori infection.
- Gastrocepin. It is considered a remedy from the reserve group for reducing acidity and is mainly used for severe diseases accompanied by hyperacid conditions.
- Gastromax. It is a combination of famotidine with enveloping agents.
Important to remember! Sodium bicarbonate (soda) is included in many medications for the treatment of high acidity. It is often used by many patients as the only treatment. This approach is unacceptable, since prolonged use of soda, in addition to a short-term decrease in acidity, causes rebound syndrome. This means that the positive effect is soon followed by an even greater increase in acidity!
Modern antacid drugs in gastroenterological practice
The possibility of a rapid therapeutic effect, primarily in the elimination (reduction of intensity) of heartburn and pain, after taking antacid drugs per os has long attracted the attention of doctors and researchers. This quality of antacid drugs distinguishes them favorably from drugs of other classes, including H2-blockers of histamine receptors and proton pump inhibitors, the use of which in the treatment of patients can significantly reduce acid formation in the stomach, but their effect occurs somewhat later, and the financial cost is much higher.
The main point of application of antacid drugs is the neutralization of hydrochloric acid secreted by the parietal cells of the gastric mucosa. According to the observations of some researchers [14], when taking antacid drugs in usual therapeutic dosages, the acidity level is no more than 5 (the drugs neutralize only excess acidity of gastric juice), however, when the acidity level decreases to 1.3–2.3, these drugs neutralize 90% gastric juice, and at a value of 3.3 - 99% gastric juice.
Antacid drugs have been used for a long time in the treatment of patients suffering from various gastroenterological diseases, primarily acid-related diseases. Currently, a large group of diseases of the upper gastrointestinal tract are classified as acid-dependent, regardless of whether the factor of acid aggression is central or only additional, leading to the occurrence and progression of these disorders. Among acid-related diseases, the most common are gastric and duodenal ulcers, gastroesophageal reflux disease (GERD), non-ulcer (functional, essential) dyspepsia (NFD), pancreatitis, ulcers associated with non-steroidal anti-inflammatory drugs (NSAIDs), Zollinger-Ellison syndrome [1 ]. Some researchers also include ulcers, which can occur with hyperthyroidism, as acid-dependent diseases [13]. In our opinion, these disorders can also include an idiopathic hypersecretory state, peptic ulcers of gastroenteroanastomosis, which occur in some patients after gastrectomy, and, to some extent, Cushing's ulcers, as well as ulcers that appear with celiac enteropathy.
When treating patients suffering from acid-dependent diseases, various antacid drugs are used, which differ from each other to a greater or lesser extent, primarily in composition, speed of onset of the therapeutic effect, duration and effectiveness of the effect. These qualities of drugs depend to some extent on their form (tablet, gel, suspension). However, most modern antacid drugs have something in common - a decrease in the concentration of hydrogen ions in the stomach, resulting from the neutralization of hydrochloric acid; in addition, the neutralizing effect causes a decrease in peptic activity. In addition, in the stomach, antacid drugs bind bile acids and lezolecithin, providing an enveloping effect. Some of the antacid drugs (in particular, those containing aluminum hydroxide) also have a cytoprotective effect, which consists in enhancing the secretion of mucus and the synthesis of prostaglandins. It also turned out that antacid drugs are able to bind epithelial growth factor and fix it in the area of the ulcer, stimulating cell proliferation, angiogenesis and tissue regeneration [1].
Taking into account the antagonistic effect of magnesium intravenously injected into the stomach on acid hypersecretion caused by calcium carbonate, products containing a mixture of calcium carbonate and magnesium oxide hydrate were created. However, such antacid drugs do not eliminate the stimulating effect of calcium carbonate on acid secretion in the stomach. In addition, antacid preparations containing calcium carbonate, when interacting with hydrochloric acid in the stomach, cause the formation of a significant amount of carbon dioxide, which leads to the appearance or intensification of flatulence, and in the presence of cardia insufficiency, including those combined with a hiatal hernia, - burps.
The stimulatory effect of some antacid drugs on gastric acid secretion is partly associated with alkalization of the gastric antrum, the release of gastrin and possibly other neurohormonal factors, and partly with the direct effect of these antacid drugs on the parietal cells of the gastric mucosa.
Repeated attempts have been made to somehow classify antacid drugs (absorbable and non-absorbable, local and systemic, anionic and cationic, combined and monocomponent). The most common types are absorbable and non-absorbable antacid drugs. The absorbable group usually includes drugs such as sodium bicarbonate (soda), basic magnesium calcium carbonate - a mixture of Mg(OH)2, 4MgCO3, H2O, magnesium oxide (burnt magnesia), basic calcium carbonate - CaCO3, a mixture of Bourget (Na sulfate, Na phosphate and Na bicarbonate), Rennie's mixture (calcium carbonate and magnesium carbonate), Tams mixture (calcium carbonate and magnesium carbonate). These antacid drugs are characterized by the relative speed of onset of the therapeutic effect (the disadvantage is the short duration of neutralization of hydrochloric acid). Typically, these drugs, having a systemic effect, increase the alkaline reserves of the plasma, changing the acid-base balance, and neutralize (with local action) hydrochloric acid in the stomach, which in some cases can lead to “acid rebound” syndrome due to the persistent appearance of hypersecretion of acid in the stomach after taking such antacid drugs [12]. In particular, these antacid drugs include calcium carbonate, which, soon after administration, begins to stimulate the secretion of acid in the stomach - accelerated neutralization of hydrochloric acid in the stomach, activates an increase in its secretion by the parietal cells of the gastric mucosa. In this regard, calcium carbonate is now very rarely used in the treatment of patients.
The group of non-absorbable antacid drugs most often includes drugs such as phosphalugel (aluminum salt of phosphoric acid), the so-called aluminum-magnesium antacid drugs (Maalox, Almagel Neo, Taltsid, Protab, Magalfil, etc.) and aluminum-magnesium antacid drugs with the addition of alginate (topalcan). A common feature of the primary action of this group of drugs (upon entry into the stomach) is the adsorbing effect on hydrochloric acid with its subsequent neutralization. Unlike absorbable antacid drugs, non-absorbable antacid drugs have a longer-lasting antisecretory (neutralizing) effect (up to 2-3 hours), do not cause changes in acid-base balance and do not lead to an increase in the pH of the gastric contents above the neutral value, without causing “acid acid” syndrome. ricochet."
Modern antacid drugs differ among themselves and in the composition of cations (magnesium, calcium, aluminum), which largely determines their basic properties (neutralizing, adsorbent, enveloping, astringent and cytoprotective effects).
Unlike monocomponent antacids, combined antacid preparations consist of several components that make them up and have different properties, depending on the composition. Sometimes aluminum-containing preparations are isolated (phosphalugel, maalox, almagel, gelusil varnish, talcid, etc.), one of the significant advantages of which, along with the neutralization of hydrochloric acid in the lumen of the stomach, is the protection of the mucous membrane of the esophagus and stomach from the effects of the acid-peptic factor. Combined antacid drugs, especially those containing aluminum, have different mechanisms of action, including a combination that neutralizes hydrochloric acid and increases the protective properties of the mucous membrane, i.e., apparently, they also have a cytoprotective effect.
When assessing the effectiveness of antacid drugs, their acid-neutralizing ability and duration of action are most often taken into account. This fact is very important: the duration of antacid exposure is one of the main factors in assessing the therapeutic effectiveness of antacid drugs used in the treatment of patients. It is known that antacid drugs, due to their ability to be adsorbed on the gastric mucosa, cause a persistent acid-neutralizing effect, allowing them to exhibit buffering properties at a level of 2.4 pH.
The acid neutralizing activity of different antacid drugs ranges from less than 20 mmol/15 ml of antacid drug to 100 mmol/15 ml [8]. The acid-neutralizing ability (activity) of antacid drugs is usually understood as the amount of a specific antacid drug in grams or mmol/l required to achieve a pH level of 50 ml of 0.1 N hydrochloric acid solution to 3.5 [4].
Among antacid drugs, those associated with the calcium carbonate group have the shortest duration of action, those associated with the magnesium group have a somewhat longer duration of action, and those associated with the phosphorus group have an even longer duration (up to 90 minutes). There are also other data on the duration of action of antacid drugs [11], in particular, those containing aluminum phosphate, which have an antacid effect due to their absorption on the gastric mucosa, which extends the duration of their buffering capacity at a pH value of 2.4 to 120 minutes.
According to a number of researchers [11], combinations of aluminum and magnesium hydroxides, as well as calcium and magnesium carbonates, mainly exhibit only neutralizing activity, which also includes accelerated passage of food through the stomach. A study of the properties of some antacid drugs [2], according to intragastric computer pH-metry, using a 3-electrode pH probe, showed that the shortest time from the start of administration of the antacid drug to the increase in pH (on average 8.9 minutes) was found in Maalox, the longest time is for Almagel (on average 13.5 minutes) compared to Remagel, Phosphalugel, Megalac; The average duration of the alkalizing effect (alkaline time - from the beginning of the pH increase to the return to the initial level) for antacid drugs ranged from 28 minutes for Almagel to 56 minutes for Maalox. At the same time, Remagel, Phosphalugel and Megalac occupied an intermediate position between Almagel and Maalox. Analysis of pH grams showed that the maximum pH values after taking various antacid drugs differed slightly.
Therapy with antacids
Antacid drugs can be successfully used in the drug therapy of all acid-dependent diseases in the following cases: 1) as monotherapy in the initial stages of these diseases; 2) as additional agents (for example, when treating patients with histamine H2 receptor blockers or prokinetics); 3) as a symptomatic means to eliminate (reduce intensity) heartburn and pain in the chest and/or epigastric region, both during the treatment of patients, combining their use with other drugs, and during the period of remission (including as therapy “ on demand"); 4) during the screening phase before the start of the intended treatment, when selecting patients for randomized studies to study the effectiveness and safety of certain medications or regimens for their use (as a rule, taking antacid drugs is allowed according to the protocols of these studies), as well as directly during timing of such studies as emergency treatment in cases where the efficacy and safety of prokinetics, H2-blockers of histamine receptors, proton pump inhibitors or so-called cytoprotective drugs are being studied.
In such cases, the undoubted advantage of antacid drugs is taken into account - the rapid elimination (reduction of intensity) of heartburn (burning) behind the sternum and / or in the epigastric region and other gastrointestinal symptoms caused by the disease itself, for which patients are being treated, taking medications and intoxication .
One of the antacid drugs that periodically attracts the attention of researchers and doctors is phosphalugel (colloidal aluminum phosphate in the form of an oral gel containing 8.8 g in one sachet). Phosphalugel is often classified as a non-absorbable antacid drug. Most of the aluminum phosphate gel is insoluble, but at a pH less than 2.5, the phosphalugel turns into water-soluble ammonium chloride, some of which is able to dissolve, after which further dissolution of the aluminum phosphate is suspended. A gradual decrease in the acidity level of gastric contents to pH 3.0 does not lead to the occurrence of “acid rebound”: the use of phosphalugel in the treatment of patients does not lead to the appearance of secondary hypersecretion of hydrochloric acid.
One of the advantages of phosphalugel is its acid-neutralizing ability depends on the level of acidity: the higher the acidity, the more active the effect of this drug [10]. An increase in pH under the influence of the drug leads to a decrease in the proteolytic activity of pepsin. The drug does not cause alkalization of gastric juice, does not limit enzymatic processes and does not disrupt the physiological conditions of the digestion process. Long-term use of the drug does not affect phosphorus metabolism. The actual effect of phosphalugel, which is in the form of hydrophilic colloidal micelles of the drug, is determined by colloidal aluminum phosphate, which has an antacid, enveloping and adsorbing effect. A small part of phosphalugel precipitates in the intestine in the form of oxides and insoluble carbonates, which enhances its protective, adsorbent and antacid effect. One gram of aluminum phosphate gel micelles, consisting of aluminum phosphate, agar gel and pectin, has a contact surface of about 1000 m², which ensures intensive communication with the walls of the digestive tract and adsorption of harmful substances. Gels of pectin and agar-agar, which are part of the drug, are involved in the formation of a mucoid, antipeptic protective layer in the gastrointestinal tract. Colloidal aluminum phosphate binds endogenous and exogenous toxins, bacteria, viruses, gases formed as a result of putrefaction and pathological fermentation throughout the gastrointestinal tract, normalizing their passage through the intestines and thereby facilitating their removal from the body of patients. The drug also reduces pain [3]. Adults and children over 6 years of age are usually prescribed 1-2 sachets 2-3 times a day immediately after meals and at night (for reflux esophagitis) or more often (for other diseases) - 1-2 hours after meals.
One of the antacid drugs that has recently also attracted the attention of doctors is hydrotalcite (rutacid, talcid), a drug with a low content of aluminum and magnesium. Among the features of the mechanism of action of this drug is the gradual release of aluminum and magnesium ions depending on the pH state of the gastric contents. Other advantages of hydrotalcite are rapid and long-term neutralization of hydrochloric acid with maintaining a pH close to normal levels, a protective effect on the gastric mucosa with a decrease in the proteolytic activity of pepsin, binding of bile acids, and also the form of release of the drug - in the form of chewable tablets that should be chewed thoroughly . When treating adult patients, hydrotalcite is usually prescribed 500–1000 mg (1-2 tablets) 3-4 times a day, 1 hour after meals and before bedtime; after errors in the diet, accompanied by the appearance of symptoms of discomfort, as well as in case of alcohol abuse - 1-2 tablets once. For children aged 6–12 years, the dosage is reduced by 2 times. The duration of treatment is determined by the general condition of the patients. It is not recommended to take this drug at the same time as drinking acidic drinks (juices, wine).
It is known that, along with dyspeptic disorders, usually associated with various diseases of the esophagus and stomach, a significant proportion of patients are bothered by flatulence, which occurs for various reasons, including in patients, according to our observations, who have been taking proton pump inhibitors for a long time. The appearance on the Russian domestic market of a new antacid water-soluble drug Almagel Neo, containing in its composition the optimal amount of aluminum hydroxide and magnesium hydroxide (compared to the previously widely known Almagel suspension, the content of the latter is increased by 3.9 times) and simethicone (defoamer) introduced into its composition , allows patients with preserved and increased gastric secretion to obtain a positive effect in eliminating symptoms of discomfort, including flatulence, in a short time (on average on the fifth to seventh day); Only in cases of severe symptoms of flatulence, treatment of patients with Almagel Neo should begin with the use of 60 ml/day [13]. The effectiveness of this drug is due to its high acid-neutralizing ability, the presence in its composition of simethicone (a surfactant that reduces the external tension of gas bubbles), which promotes the natural release of intestinal gases and their absorption, which to a certain extent prevents the occurrence of stool retention (constipation) and flatulence , reduces the likelihood of belching. The presence of neo sorbitol in Almagel allows it to be used in the treatment of patients who, along with one of the acid-related diseases, also have diabetes mellitus. The usual dosages for prescribing this drug to patients are: orally for adults, 1 sachet or 2 dosage spoons 4 times a day, 1 hour after meals and at night; For children over 10 years of age, the dosage of the drug is determined by the attending physician (taking into account the body weight and condition of the child).
There are different options for prescribing antacid drugs to patients for various diseases, but most often antacid drugs are prescribed in the following cases: with the so-called “on-demand” therapy for the rapid elimination (reduction of intensity) of the symptoms of dyspepsia, especially heartburn and pain (at any time of the day) ; during a course of treatment 30–40 minutes before or 30–60 minutes after a meal (if necessary and before bedtime) as monotherapy or in complex treatment, in combination, first of all, with prokinetics and/or with H2-blockers of histamine receptors (the frequency and duration of taking antacid medications are determined by the general condition of the patients). The positive effect of antacid drugs in eliminating pain in the chest and/or epigastric region and/or heartburn (burning) in itself indicates the presence of an acid-dependent disease in the patient. Most often, as observations show, antacid drugs may be necessary in the treatment of patients suffering from peptic ulcer disease, chronic pancreatitis, GERD and/or NFD, which can be combined with chronic hyperacid or normacid gastritis, and is also possible in patients with NFD syndrome without morphological signs of gastritis.
As our observations have shown, it is most advisable to use antacid drugs in the following cases. For peptic ulcer disease associated with Helicobacter pylori (HP), after eradication therapy when pain and/or dyspeptic disorders, especially heartburn, appear in patients. However, due to the adsorbing ability of antacid drugs, their use directly during Helicobacter pylori eradication therapy is not justified: during this period, patients take quite a lot of tablets or capsules - 6 times a day the basic drug (proton pump inhibitor, ranitidine or bismuth drug) in combination with 2 antibiotics (first-line therapy) or 13 times a day 4 drugs (second-line therapy), as the likelihood of a decrease in the effectiveness of both antibiotics and the basic drug(s) increases. Taking into account the number of medications used by patients during the day and necessary to obtain the eradication effect, i.e., the destruction of Helicobacter pylori (HP), in the case of additional prescription of antacid drugs, the number of tablet forms of drugs will exceed the specified number of drug doses (taking into account dosages), more than 6 and 13 times a day in first- and second-line therapy, respectively.
For peptic ulcers not associated with HP, antacid drugs can be successfully used as independent therapy for newly diagnosed, uncomplicated duodenal ulcers (with small ulcers), as well as as additional therapy for gastric and duodenal ulcers to H2 - histamine receptor blockers, or in on-demand therapy for them or proton pump inhibitors. The success of treating patients largely depends on the depth of the ulcer.
When comparing the results of a 4-week treatment of 2 groups of patients suffering from uncomplicated duodenal ulcer (one of the groups was treated with various antacid drugs in “liquid” form or in tablet form, 4-6 times a day, which had different neutralizing abilities - from 120 to 595 mEq of H+ anions per day, another group of patients was treated in therapeutic doses with H2-blockers of histamine receptors [7]), there were no significant differences in the time of disappearance of clinical symptoms and healing of ulcers. Another study [6] compared the results of treatment of 42 patients treated with phosphalugel, 11 g of aluminum phosphate gel 3 times a day (after meals) for 4 weeks, and treatment of 49 patients treated with ranitidine, 150 mg 2 times a day, also in for 4 weeks, showed the following: healing of duodenal ulcers was noted in 60 and 55% of cases, respectively. According to another study [7], based on an analysis of the results of a 6-week treatment of 153 patients who received aluminum phosphate (1 sachet = 11 g of gel) 5 times a day, ulcer healing was established in 65% of cases.
Depending on the stage of treatment of GERD, antacid drugs can be effectively used in the following cases: as the main drug in some patients with endoscopically negative GERD and with GERD in the stage of mild reflux esophagitis (with minimally expressed symptoms); in combination with H2-blockers of histamine receptors in the course treatment of patients with GERD in the stage of mild or moderate reflux esophagitis, as well as during on-demand therapy; during a course of treatment of patients with GERD in the stage of erosive reflux esophagitis in combination with H2-blockers of histamine receptors, in on-demand therapy in combination with continuous treatment of patients with proton pump inhibitors (during exacerbation of the disease); during a course of treatment of patients with GERD in the stage of peptic ulcer of the esophagus in combination with H2-blockers of histamine receptors or in on-demand therapy (while treating patients with proton pump inhibitors).
To improve the condition of patients, it is advisable to use antacid drugs in the treatment of patients suffering from other diseases: in particular, with erosive and ulcerative lesions of the stomach and duodenum associated with non-steroidal anti-inflammatory drugs, with erosive and ulcerative lesions of the upper gastrointestinal tract, the occurrence of which possible with decompensated liver cirrhosis, with peptic ulcer disease combined with celiac enteropathy, and with Zollinger-Ellison syndrome.
During the treatment of patients with the listed diseases, it is advisable to use antacid drugs during a course of therapy in combination with H2-blockers of histamine receptors (in on-demand therapy and with proton pump inhibitors).
The use of antacid drugs is useful, as observations have shown, in the treatment of patients with acute gastritis (as an additional adsorbent agent for various types of acute gastritis); as an additional therapy (to H2-blockers of histamine receptors or to proton pump inhibitors) for Cushing's ulcers; in the treatment of patients with peptic ulcers of gastroenteroanastomosis and patients with chronic pancreatitis. Antacids are used in combination with H2-blockers or proton pump inhibitors as on-demand therapy.
It is advisable to use antacid drugs in the treatment of patients with functional bowel diseases in order to eliminate pain and/or discomfort. It was shown [9] that one dose of aluminum phosphate gel with a volume of 100 to 300 ml, prescribed per os, just before taking a dose of radiostrontium 85Sr, reduced the absorption of the latter by 87.5%, while a dosage of 100 ml of aluminum phosphate gel was just as effective , as well as 300 ml, which indicates other possibilities for using antacid drugs.
It is known that aluminum phosphate gel, which is a combination of an antacid and substances that cover and protect the mucous membrane from the pathological effects of acid and bile acids, helps eliminate (reduce) their “irritating” (pathological) effect on the mucous membrane of the esophagus and stomach, which makes it possible to recommend short-term the use of this drug in pregnant women or during lactation after childbirth [5]. The same advantages of phosphalugel (cytoprotective effect of the drug) protect the mucous membrane from damage and from the effects of alcohol [4].
As a symptomatic (additional) remedy for eliminating (reducing the intensity) symptoms of dyspepsia, antacid drugs can also be used in the treatment of patients with organic dyspepsia of various etiologies (for example, before surgical treatment of patients, if necessary, and after it), as well as to eliminate symptoms of discomfort in people who consider themselves healthy.
Features of prescribing antacids
When prescribing antacid drugs, it is necessary to take into account the mechanism(s) of their action and the symptoms of diseases observed in specific patients (constipation, diarrhea, etc.). In particular, in the presence of diarrhea (as additional means, if necessary), it is advisable to treat patients with antacid drugs containing aluminum (almagel, phosphalugel, rutacid, talcid); for constipation - antacid medications that contain magnesium (Gelusil Lac, Gastal, etc.).
It is known that antacid drugs (when entering the body of patients) have an adsorbing ability, due to which it is possible to reduce the activity and bioavailability of some medications taken by patients (for example, H2-blockers of histamine receptors, non-steroidal anti-inflammatory drugs, antibiotics, etc.). Therefore, when prescribing antacid drugs in combination with other drugs, it is advisable to recommend that patients observe the time interval between taking antacid drugs and other drugs (before or after, about 2-2.5 hours), i.e., indicate the time patients take specific drugs during the day .
According to our observations, the effect of taking antacid drugs produced in the form of gels or suspensions (compared to tablet forms) occurs faster, although the tablet form seems somewhat more convenient for storage (especially when traveling).
When deciding on the use of antacid drugs, especially long-term (in high dosages), it is necessary to take into account the possibility of side effects. Side effects that are possible in some patients while taking antacid drugs largely depend on the individual characteristics of the patient, the dosage of antacid drugs and the duration of their use. Constipation or diarrhea (depending on the particular antacid drug used in the treatment of patients) are the most common side effects that occur in patients while taking antacid drugs. A significant increase in the dosage of antacid drugs is the main cause of constipation or diarrhea, and long-term, uncontrolled use is the main cause of metabolic disorders.
In particular, one of the features of the action of magnesium-containing antacid drugs is an increase in intestinal motor function, which can lead to normalization of stool, but if taken in excess, to the development of diarrhea. An overdose of magnesium-containing antacid drugs (an increase in Mg+++ ions in the patient’s body) increases the magnesium content in the patient’s body, which can cause bradycardia and/or insufficiency of renal function.
Antacid drugs containing calcium, in case of overdose, cause an increase in Ca++ in the body of patients (the occurrence of hypercalcemia), which can lead to the so-called “alkaline” syndrome in patients suffering from urolithiasis, which, in turn, contributes to increased formation of stones. A decrease in the production of parathyroid hormone can lead to a delay in the excretion of phosphorus, an increase in the content of insoluble calcium phosphate and, consequently, to calcification of the body tissues of patients and the occurrence of nephrocalcinosis.
The level of aluminum absorption may be different for different drugs, which must be taken into account when determining the possible risk of side effects due to the fact that antacid drugs containing aluminum can cause hypophosphatemia in some patients, especially with prolonged use; in renal failure - encephalopathy, osteomalacia (with an aluminum level of more than 3.7 µmol/l), clinical symptoms considered characteristic of poisoning (with an aluminum concentration of more than 7.4 µmol/l). It is also necessary to take into account the fact that the lower toxicity of aluminum phosphate A1PO4, compared to aluminum hydroxide A1(OH)3, is due to its greater resistance to dissolution and the formation of neutral complexes in the presence of acids usually contained in food, which indicates the lower toxicity of the phosphate aluminum
As a rule, the occurrence of side effects can be avoided if, when prescribing antacid drugs, the mechanism of their action, the condition of specific patients is taken into account, and, in addition, if detailed explanatory work is carried out with patients before prescribing antacid drugs.
For questions regarding literature, please contact the editor.
Yu. V. Vasiliev, Doctor of Medical Sciences, Professor
Central Research Institute of Gastroenterology, Moscow
Diet
During the period of exacerbation (when pain is tormented), you need to endure at least a week on dietary soups without frying, porridges, stewed or boiled potatoes, small portions of boiled meat. During this period, you can drink teas, jelly, compotes (not sour), juices - only pumpkin, and then only a little. Alcohol, smoked, spicy, salty foods, carbonated drinks, sour fresh vegetables and fruits are completely excluded. The bread is stale, optimally dried. All food is neither hot nor cold (the best option is warm).
During the period of remission, the diet expands. The patient can eat low-fat varieties of fish (hake, cod, pink salmon), meat (rabbit, chicken, beef); The side dish is porridge, potatoes, maybe pasta, but not much and not often. Eggs, fresh non-acidic fruits and dairy products are also included in the diet. It is useful to drink fresh cabbage juice. Restrictions on the intake of fatty foods, smoked peppers, alcohol and strong teas and coffee remain.
Menu for the week
The diet used for stomach acidity is based on food that is gentle on the functions of the gastrointestinal tract. During such a diet, fatty and spicy foods, sausages, pickles, marinades, carbonated drinks, and fast foods are excluded from consumption. The basis of the diet consists of foods that reduce acidity levels.
Day 1−2:
- Breakfast: ground buckwheat porridge (with milk), cottage cheese soufflé, herbal tea.
- Lunch: soft-boiled egg, vegetables.
- Lunch: light vegetable soup, meatballs, carrot puree, jelly.
- Dinner: fish cutlets, pasta.
- Before bed: milk or kefir.
Day 3−4:
- Breakfast: oatmeal, carrot puree, tea.
- Lunch: pancakes.
- Lunch: zucchini soup, steamed cutlets, pasta, compote.
- Dinner: lazy dumplings, plum soufflé.
- Before bed: milk or kefir.
Day 5−6:
- Breakfast: baked omelette, avocado toast, tea.
- Lunch: carrot-apple soufflé.
- Lunch: rice soup (milk-based), boiled meat, jelly.
- Dinner: mashed potatoes, a little spinach, low-fat cottage cheese.
- Before bed: milk or kefir.
Day 7:
- Breakfast: semolina porridge, dry cookies, tea with milk.
- Lunch: egg soufflé.
- Lunch: chicken soup with croutons, baked apples with honey, fresh vegetable salad, dried fruit compote.
- Dinner: baked chicken with rice, boiled potatoes, stewed vegetables.
- Before bed: milk or kefir.
Don't go to bed on a full stomach. Lying down immediately after eating can cause stomach acid to move back up the tract instead of down. As a result, this only contributes to the stimulation of an increase in acidity levels.
Folk remedies
The patient can feel better with the help of infusions and decoctions based on medicinal herbs for high stomach acidity.
- A decoction based on mint, fennel herb and caraway seeds. It is necessary to take all the ingredients in equal proportions, pour boiling water over them and consume 100 ml 2 times a day before meals.
- Chamomile tea. It is enough to pour 150 ml of boiling water 1 tbsp. l. chamomile herbs purchased at a pharmacy. Leave for half an hour. Take before meals. This tea stabilizes digestion.
- A decoction of St. John's wort herb. Mix 50 g of herb with a glass of boiling water. Consume strictly before meals.
- Mint decoction. To prepare the product you will need a glass of mint and 500 ml of boiling water. Bring the resulting mixture to a boil over low heat and cook for 10 minutes, then leave the broth for half an hour. It should be taken at least 6 times a day, half a glass. The decoction has a calming effect on the gastric mucosa and enhances metabolism.
It is not recommended to use ginger, plantain and rosehip during treatment. They contain substances that irritate the gastric mucosa.
Prevention
To prevent increased acidity, you need to follow a number of rules. They are not heavy and do not require serious effort, but will help keep your stomach acidity in order. Of course, proper nutrition comes first in importance. The amount of proteins, carbohydrates, fats, fiber, vitamins must be balanced. It is important to follow a meal schedule. The last meal is 3 hours before bedtime. If we are talking about light meals, for example, low-fat dairy products, you can eat them even 30 minutes before bedtime.
Try to avoid hunger and mono-diets, do not overeat, but eat dry. It is better to avoid fried, fatty foods and dishes. Eat warm food, not hot or cold. It is better to give up bad habits (alcohol, smoking) if you decide to get your acid-base balance in order. Observe the storage conditions and terms of food products.
It is necessary to maintain individual hygiene and sanitize the mouth in a timely manner. Treat any illnesses on time, since all organs and systems in the body are connected. And the most unexpected disease can affect the balance of the stomach. Any medications should be taken only as prescribed by a doctor. Try to think positively and avoid stress; it is with psycho-emotional stress that most gastrointestinal diseases begin.
How to maintain pH balance in the body
Sports - two hours a week
During intense muscle work during sports, the body removes excess acid through the skin and kidneys. But do not overdo it, because when overloaded, lactic acid is formed in the body, which disrupts the acid-base balance (the signal for the onset of acidification is muscle pain). On average, you should train for 40 minutes three times a week. Instead of heavy weight training, choose gymnastics, running, yoga, swimming or cycling.
Keep your plate balanced
In order for the pH to remain normal, the plate must contain 80% alkaline foods (for example: vegetables, fruits, berries, herbs, milk) and 20% acidic foods (meat, fish, bread, beans, etc.).
Twice a day - salted water
Salt (sodium) is needed to lower the level of acids in the body's cells and eliminate acid in the urine. Drink two glasses of salted water a day (a pinch of sea salt per liter of water).
Do breathing exercises three times a day
Lack of oxygen in cells and tissues leads to disruption of blood pH. Breathing exercises will help saturate the body with oxygen. Take short, shallow breaths through your nose and exhale sharply and deeply through your mouth. Repeat these breaths every five seconds for two to three minutes.
Once a week - avocado
It is a source of omega-3 fatty acids, calcium, potassium, magnesium and sodium, which in the set normalize pH levels.
What to do if you are bitten by a tick
pH balance tests
Tests of urine, saliva, gastric juice, and blood will help you find out about the state of your acid-base balance. The first two analyzes can be carried out at home using test strips (from 23 UAH) - the use and interpretation of the results are indicated in the instructions. The remaining two tests are prescribed by the doctor according to indications: abdominal pain, anemia, etc.