Compound
- 1 capsule of Irunin contains 100 mg of itraconazole . Additional components: methyl parahydroxybenzoate, granulated sugar, hypromellose, titanium dioxide, methacrylic acid in copolymer form, sucrose, propyl parahydroxybenzoate, gelatin, quinoline yellow dye.
- 1 vaginal tablet Irunin contains 200 mg of itraconazole . Additional components: sodium lauryl sulfate, starch, lactose, talc, povidone, magnesium stearate.
Release form
Yellow capsules, inside are spherical white pellets.
- 5 pieces in a cell package - 2, 3 or 1 package in a cardboard box.
- 6 pieces in a cell package - 2, 3 or 1 package in a cardboard box.
- 7 pieces in a cell package - 2, 3 or 1 package in a cardboard box.
- 10 pieces in a cell package - 2, 3 or 1 package in a cardboard box.
- 14 pieces in a cell package - 2, 3 or 1 package in a cardboard box.
- 15 pieces in a cell package - 2, 3 or 1 package in a cardboard box.
- 5, 6, 7, 10, 14 or 15 pieces in a glass jar - 1 jar in a cardboard box.
White vaginal tablets, ring-shaped.
- 10 pieces in a cell package - 1 package in a cardboard box.
Pharmacodynamics and pharmacokinetics
Pharmacodynamics
Itraconazole is a triazole that exhibits potent antifungal activity. Blocks P450 cytochrome-dependent enzymes in fungal cells, which suppresses the biosynthesis of ergosterol in their walls. Has a broad spectrum of antifungal activity. Effective against Sporothrix schenckii, Paracoccidioides brasiliensis, Blastomyces dermatitidis, Cryptococcus neoformans, Histoplasma capsulatum, Malassezia furfur, Coccidioides immitis, as well as fungi of the genus Candida, Aspergillus, Microsporum, Epidermophyton, Trichophyton.
Pharmacokinetics
Therapeutic concentrations in skin tissues remain for 15-30 days after completion of a monthly course of therapy. The drug is detected in the nails 7 days after the start of therapy and remains in them for another six months after completion of the 3-month course of treatment.
When administered intravaginally, a local effective concentration of the active substance is achieved. Systemic absorption is minimal.
Pharmacodynamics
Inhibits the synthesis of ergosterol in the cell membrane of fungi, which determines the antifungal effect of the drug.
Itraconazole is active against infections caused by dermatophytes (Trichophyton spp., Microsporum spp., Epidermophyton floccosum), yeast-like fungi and yeasts (Cryptococcus neoformans, Pityrosporum spp., Candida spp., including C. albicans, C. glabrata and C. krusei) ; Aspergillus spp., Histoplasma spp., Paracoccidioides brasiliensis, Sporothrix schenckii, Fonsecaea spp., Cladosporium spp., Blastomyces dermatitidis, as well as other yeasts and molds.
Indications for use
Indications for use of capsules
- mycoses of the skin, eyes, oral mucosa;
- onychomycosis , caused by dermatophytes or yeasts ;
- vulvovaginal candidiasis;
- systemic mycoses (including systemic sporotrichosis, candidiasis, aspergillosis, histoplasmosis, cryptococcosis, blastomycosis, paracoccidioidosis , and other rarely reported systemic mycoses).
Indications for use of vaginal tablets
- vulvovaginal candidiasis.
Irunin®
— Women of childbearing age taking Irunin® must use adequate contraception throughout the entire course of treatment until the onset of the first menstruation after its completion.
— When studying the intravenous dosage form of the drug Itraconazole, a transient asymptomatic decrease in left ventricular ejection fraction was noted, which normalized until the next infusion of the drug.
— Itraconazole has been found to have a negative inotropic effect. Cases of heart failure associated with taking Irunin have been reported. Irunin ® should not be taken by patients with chronic heart failure or with a history of this disease, unless the possible benefit significantly outweighs the potential risk.
- Calcium channel blockers may have a negative inotropic effect, which may enhance this effect of itraconazole; itraconazole may decrease the metabolism of calcium channel blockers. Caution should be used when taking itraconazole and calcium channel blockers concomitantly.
- In patients with renal impairment, the bioavailability of itraconazole may be reduced, which may require dose adjustment.
— With reduced gastric acidity, the absorption of itraconazole is impaired. Patients taking antacid medications (for example, aluminum hydroxide) are recommended to use them no earlier than 2 hours after taking Irunin® capsules. Patients with achlorhydria or using H2-histamine blockers or proton pump inhibitors are recommended to take Irunin® capsules with acidic drinks.
— In very rare cases, when using Irunin®, severe toxic liver damage developed, including cases of acute liver failure with a fatal outcome. This occurred in patients who already had liver disease, as well as in patients receiving other drugs that have hepatotoxic effects. Several such cases occurred in the first month of therapy, and some in the first week of treatment. In this regard, it is recommended to regularly monitor liver function in patients receiving itraconazole therapy.
- Treatment should be discontinued if neuropathy occurs, which may be associated with taking Irunin®.
— There is no evidence of cross-hypersensitivity to itraconazole and other azole antifungals. Irunin® capsules should be administered with caution to patients with hypersensitivity to other azoles.
— In patients with compromised immunity (AIDS, after organ transplantation, neutropenia), an increase in the dose of Irunin® may be required.
Contraindications
Contraindications for capsules
- Co-administration of Dofetilide, Astemizole, Terfenadine, cisapride, Mizolastine, Pimozide, Quinidine, Lovastatin, Simvastatin, Triazolam or midazolam .
- Allergy to itraconazole and other components of the drug.
- Pregnancy and lactation.
Contraindications for vaginal tablets Irunin
- Pregnancy and lactation.
- Allergy to itraconazole and other components of the drug.
Side effects
Possible side effects when taking capsules
- Reactions from the circulatory system: congestive heart failure, peripheral edema, pulmonary edema .
- Reactions from nervous activity: headache, dizziness , peripheral neuropathy .
- Digestive reactions: constipation , vomiting , nausea , cholestatic jaundice, hepatitis, abdominal pain, increased liver enzymes.
- Other reactions: hypokalemia , hair loss , dysmenorrhea .
- Allergic reactions: angioedema , rash , urticaria , itching , Stevens-Johnson syndrome .
Possible side effects when using vaginal tablets
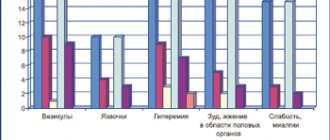
When using vaginal tablets, the following local symptoms cannot be ruled out: itching, rash on the skin of the genital organs, burning sensation in the vagina. These phenomena do not require discontinuation of use of the drug.
Irunin 100 mg caps No. 6
Dosage
0.1 g
Active substance
Itraconazole
Manufacturer
Veropharm JSC (Russia)
Shelf life
3 years
Storage conditions
At a temperature not exceeding 25 °C
Registration certificate number
R N001638/01 dated 08/26/2014
Description of the dosage form
Yellow capsules No. 0. The contents of the capsules are spherical microgranules from light yellow to yellowish-beige.
Pharmacokinetics
Maximum bioavailability of itraconazole is observed when capsules are taken orally immediately after a heavy meal. Cmax in plasma is achieved within 3-4 hours after oral administration. Elimination from plasma is 2-phase with a final half-life of 1 to 1.5 days. With long-term use, equilibrium concentration is achieved within 1–2 weeks. The concentration of itraconazole in plasma 3-4 hours after taking the drug is 0.4 mcg/ml (100 mg once daily), 1.1 mcg/ml (200 mg once daily) and 2.0 mcg/ml ( 200 mg 2 times a day). Plasma protein binding - 99.8%. The accumulation of the drug in keratin tissues, especially in the skin, is approximately 4 times higher than the accumulation in the blood plasma, and the rate of its elimination depends on the regeneration of the epidermis. In contrast to plasma concentrations, which are undetectable 7 days after cessation of therapy, therapeutic skin concentrations persist for 2-4 weeks after cessation of a 4-week course of treatment. Itraconazole is detected in nail keratin as early as 1 week after the start of treatment and persists for at least 6 months after completion of a 3-month course of therapy. Itraconazole is also detected in sebum and, to a lesser extent, in sweat.
Itraconazole is well distributed in tissues that are susceptible to fungal infections. Concentrations in the lungs, kidneys, liver, bones, stomach, spleen and muscles were 2-3 times higher than the corresponding plasma concentrations. Therapeutic concentrations in vaginal tissues remain for another 2 days after the end of a 3-day course of treatment at a dose of 200 mg per day, and 3 days after the end of a 1-day course of treatment at a dose of 200 mg 2 times a day.
Itraconazole is metabolized by the liver to form a large number of metabolites. One such metabolite is hydroxyitraconazole, which has comparable antifungal activity to itraconazole in vitro.
. Antifungal concentrations of the drug determined by microbiological methods were approximately 3 times higher than those measured by HPLC. Excretion in feces ranges from 3 to 18% of the dose. Approximately 35% of the dose is excreted as metabolites in the urine within 1 week.
Pharmacodynamics
Inhibits the synthesis of ergosterol in the cell membrane of fungi, which determines the antifungal effect of the drug.
Itraconazole is active against infections caused by dermatophytes (Trichophyton spp., Microsporum spp., Epidermophyton floccosum)
, yeast-like fungi and yeasts
(Cryptococcus neoformans, Pityrosporum spp., Candida spp.
, including
C. albicans, C. glabrata
and
C. krusei
);
Aspergillus spp., Histoplasma spp., Paracoccidioides brasiliensis, Sporothrix schenckii, Fonsecaea spp., Cladosporium spp., Blastomyces dermatitidis
, as well as other yeasts and molds.
Contraindications
Hypersensitivity to the drug or its components.
Carefully:
childhood;
severe heart failure;
liver diseases (including those accompanied by liver failure);
simultaneous use with terfenadine, astemizole, mizolastine, cisapride, dofetilide, quinidine, pimozide, metabolized by the CYP3A4 enzyme, HMG-CoA reductase inhibitors (simvastatin and lovastatin), triazolam and midazolam (see also section “Interactions with other drugs”) .
Use during pregnancy and breastfeeding
Based on the results of preclinical studies and due to the fact that studies on the use of itraconazole in pregnant women have not been conducted, itraconazole should be prescribed to pregnant women only for life-threatening systemic mycoses when the potential benefit to the woman justifies the possible risk to the fetus.
Women of childbearing age taking Irunin must use adequate methods of contraception throughout the entire course of treatment until the onset of the first menstruation after its completion.
Since small amounts of itraconazole are excreted in breast milk, the expected benefit of taking itraconazole must be weighed against the risk to the baby during breastfeeding. If in doubt, do not breastfeed.
Directions for use and doses
Inside,
immediately after eating, swallowing whole.
Doses and duration of administration for a number of indications are presented in Table 1.
Table 1
| Indication | Dose | Duration, days |
| Vulvovaginal candidiasis | 200 mg 2 times a day | 1 |
| or 200 mg 1 time per day | 3 | |
| Pityriasis versicolor | 200 mg 1 time per day | 7 or 15 |
| Dermatomycosis of smooth skin | 200 mg 1 time per day | 7 |
| 100 mg 1 time per day | 15 | |
| Fungal keratitis | 200 mg 1 time per day | 21 |
| Oral candidiasis | 100 mg 1 time per day | 15 |
Lesions of highly keratinized areas of the skin such as hands and feet require additional treatment for 15 days at 100 mg/day.
The oral bioavailability of itraconazole may be reduced in some immunocompromised patients, such as patients with neutropenia, AIDS, or organ transplant recipients. Therefore, a doubling of the dose may be required.
Onychomycosis
Pulse therapy
(see table 2).
One course of pulse therapy - 2 caps. Irunin 2 times a day (200 mg 2 times a day), daily, for 1 week.
For the treatment of fungal infections of the nail plates of the hands, two courses are recommended. Three courses are recommended for the treatment of fungal infections of the nail plates of the feet. The interval between courses during which you do not need to take the drug is 3 weeks.
Clinical results will become obvious after the end of treatment, as the nails grow back.
table 2
| Localization of onychomycosis | weeks | ||||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | |
| Foot lesions with or without nail plate lesions | 1st year | Weeks free from taking Irunin | 2nd year | Weeks free from taking Irunin | 3rd year | ||||
| Damage to the nail plates of the hands | 1st year | Weeks free from taking Irunin | 2nd year | — | — | ||||
Or
Continuous treatment:
2 caps. per day (200 mg 1 time per day) for 3 months. The removal of Irunin from the skin and nail tissue is slower than from plasma. Thus, optimal clinical and mycological effects are achieved 2–4 weeks after the end of treatment for skin infections and 6–9 months after the end of treatment for nail infections.
Systemic mycoses
(Recommended dosages and duration of treatment vary depending on the type of infection - see Table 3)
Table 3
| Indication | Dose | Average duration | Note |
| Aspergillosis | 200 mg 1 time per day | 2–5 months | Increase dose to 200 mg twice daily for invasive or disseminated disease |
| Candidiasis | 100–200 mg 1 time per day | from 3 weeks to 7 months | |
| Cryptococcosis (except meningitis) | 200 mg 1 time per day | from 2 months to 1 year | Maintenance therapy - 200 mg 1 time per day |
| Cryptococcal meningitis | 200 mg 2 times a day | ||
| Histoplasmosis | from 200 mg 1 time per day to 200 mg 2 times per day | 8 months | |
| Sporotrichosis | 100 mg 1 time per day | 3 months | |
| Paracoccidioidomycosis | 100 mg 1 time per day | 6 months | |
| Chromomycosis | 100–200 mg 1 time per day | 6 months | |
| Blastomycosis | from 100 mg 1 time per day to 200 mg 2 times per day | 6 months |
Side effects
The most commonly reported adverse reactions associated with the use of itraconazole were gastrointestinal reactions such as dyspepsia, nausea, abdominal pain and constipation. In addition, the following were noted: anorexia, headache, fatigue, reversible increase in liver enzyme activity, cholestatic jaundice, hepatitis, menstrual irregularities, dizziness and allergic reactions (such as itching, rash, urticaria and angioedema), peripheral neuropathy, Stevens syndrome - Johnson, alopecia, hypokalemia, edema, congestive heart failure and pulmonary edema, dark urine color, hypercreatininemia.
In very rare cases, when using Irunin, severe toxic damage to the liver developed, incl. cases of acute liver failure with fatal outcome.
Interaction
1. Drugs that affect the metabolism of itraconazole
The simultaneous use of itraconazole with rifampicin, rifabutin and phenytoin, which are potential inducers of liver enzymes, is not recommended. Interaction studies with other hepatic enzyme inducers such as carbamazepine, phenobarbital and isoniazid have not been conducted, but similar results can be expected.
Since itraconazole is primarily metabolized by the enzyme CYP3A4, potential inhibitors of this enzyme may increase the bioavailability of itraconazole (ritonavir, indinavir, clarithromycin and erythromycin).
2. Effect of itraconazole on the metabolism of other drugs
Itraconazole may inhibit the metabolism of drugs converted by the CYP3A4 enzyme. The result of this may be an intensification or prolongation of their action, incl. and side effects.
After cessation of treatment, plasma levels of itraconazole decrease gradually depending on the dose and duration of treatment (see section "Pharmacokinetics").
Due to this reason, the following should not be prescribed simultaneously with itraconazole:
terfenadine, astemizole, mizolastine, cisapride, triazolam and oral midazolam, dofetilide, quinidine, pimozide, HMG-CoA reductase inhibitors (simvastatin and lovastatin).
CCBs may have a negative inotropic effect, which may enhance the same effect exhibited by itraconazole. When taking itraconazole and CCBs simultaneously, caution must be exercised because CCB metabolism may be reduced.
In case of simultaneous administration with itraconazole, it is necessary to monitor their plasma concentrations, side effects and, if necessary, reduce the dose of the following drugs: oral anticoagulants; HIV protease inhibitors such as ritonavir, indinavir, saquinavir; some antitumor drugs, such as rose vinca alkaloids, busulfan, docetaxel, trimetrexate; CCBs metabolized by the CYP3A4 enzyme (dihydropyridine and verapamil); some immunosuppressive drugs: cyclosporine, tacrolimus, sirolimus; other drugs: digoxin, carbamazepine, buspirone, alfentanil, alprazolam, brotizolam, rifabutin, methylprednisolone, ebastine, reboxetine.
No interaction of itraconazole with zidovudine and fluvastatin was detected. There was no effect of itraconazole on the metabolism of ethinyl estradiol and norethisterone.
In vitro studies
demonstrated the lack of competition between itraconazole and drugs such as imipramine, propranolol, diazepam, cimetidine, indomethacin, tolbutamide and sulfamethazine in binding to plasma proteins.
Overdose
No data available.
Treatment:
In case of accidental overdose, supportive measures should be used. During the first hour, gastric lavage and, if necessary, administration of activated charcoal. Itraconazole is not eliminated by hemodialysis. There is no specific antidote.
special instructions
Since there is insufficient clinical data on the use of itraconazole capsules in children, it is recommended that itraconazole be used only if the potential benefit outweighs the potential risk.
In a study of the intravenous dosage form of the drug itraconazole, conducted on healthy volunteers, a transient asymptomatic decrease in left ventricular ejection fraction was noted, which normalized until the next infusion of the drug.
Itraconazole has been found to have a negative inotropic effect. Cases of heart failure associated with taking Irunin have been reported. The drug should not be taken by patients with chronic heart failure or a history of this disease, unless the possible benefit significantly outweighs the potential risk. When individually assessing the balance of benefit and risk, factors such as the severity of the indication, dosage regimen and individual risk factors for heart failure should be taken into account. Risk factors include the presence of heart diseases such as coronary artery disease or valve disease; serious lung diseases (obstructive pulmonary lesions); renal failure or other diseases accompanied by edema. Such patients should be informed about the signs and symptoms of heart failure. Treatment should be carried out with caution, and the patient must be monitored for symptoms of congestive heart failure. If they appear, taking Irunin® must be stopped.
The clinical significance of these data for oral dosage forms is unknown.
CCBs may have a negative inotropic effect, which may enhance this effect of itraconazole; itraconazole may reduce the metabolism of CCBs. Caution should be exercised when taking itraconazole and CCBs concomitantly.
With reduced gastric acidity, the absorption of itraconazole is impaired. Patients taking antacid medications (for example, aluminum hydroxide) are recommended to use them no earlier than 2 hours after taking Irunin capsules. Patients with achlorhydria or using H2-histamine receptor blockers or proton pump inhibitors are recommended to take Irunin capsules with acidic drinks.
In very rare cases, when using Irunin, severe toxic damage to the liver developed, incl. cases of acute liver failure with fatal outcome. In most cases, this occurred in patients who already had liver disease, in patients receiving therapy for systemic indications, with other serious medical conditions, and in patients receiving other drugs that have hepatotoxic effects. Some patients had no obvious risk factors for liver damage. Several such cases occurred in the first month of therapy, and some in the first week of treatment. In this regard, it is recommended to regularly monitor liver function in patients receiving itraconazole therapy. Patients should be advised to contact their physician immediately if symptoms suggestive of hepatitis occur, such as anorexia, nausea, vomiting, weakness, abdominal pain, and dark urine; if such symptoms occur, therapy should be discontinued immediately and a function test performed. liver. Patients with elevated liver enzymes or active liver disease, or who have suffered liver toxicity while taking other drugs, should not be treated with Irunin, unless the expected benefit justifies the risk of liver damage. In these cases, it is necessary to monitor the activity of liver enzymes during treatment.
In patients with liver cirrhosis, T1/2 of itraconazole is slightly increased, and the bioavailability of the drug when taken orally is slightly reduced. In this case, dose adjustment may be necessary. When taking for more than 1 month, monitoring of liver function is necessary.
If renal function is impaired: in patients with renal failure, the bioavailability of itraconazole may be reduced. In this case, dose adjustment may be necessary.
Treatment should be discontinued if neuropathy occurs, which may be associated with taking Irunin capsules.
There is no evidence of cross-hypersensitivity to itraconazole and other azole antifungals. Irunin capsules should be administered with caution to patients with hypersensitivity to other azoles.
In patients with compromised immunity (AIDS, condition after organ transplantation, neutropenia), an increase in dose may be required.
Impact on the ability to drive a car and operate machinery
Not observed.
Pharmgroups
Antifungal agents
Pharmaceutical actions
broad spectrum antifungal
Instructions for use of Irunin (Method and dosage)
Irunin capsules, instructions for use
The medicine is taken orally. Usually prescribed to take 100 mg once a day or 200 mg up to 2 times a day. The duration of treatment depends on the type of pathogen.
Vaginal tablets (suppositories) Irunin, instructions for use
This dosage form is administered intravaginally, immediately before bedtime, in a horizontal position of the body with legs bent at the knees.
The duration of treatment is usually 1-2 weeks. On the recommendation of a doctor, a repeat cycle of treatment is allowed.
Directions for use and doses
Inside, immediately after eating, swallowing whole.
Doses and duration of administration for a number of indications are presented in Table 1.
Table 1
| Indication | Dose | Duration, days |
| Vulvovaginal candidiasis | 200 mg 2 times a day | 1 |
| or 200 mg 1 time per day | 3 | |
| Pityriasis versicolor | 200 mg 1 time per day | 7 or 15 |
| Dermatomycosis of smooth skin | 200 mg 1 time per day | 7 |
| 100 mg 1 time per day | 15 | |
| Fungal keratitis | 200 mg 1 time per day | 21 |
| Oral candidiasis | 100 mg 1 time per day | 15 |
Lesions of highly keratinized areas of the skin such as hands and feet require additional treatment for 15 days at 100 mg/day.
The oral bioavailability of itraconazole may be reduced in some immunocompromised patients, such as patients with neutropenia, AIDS, or organ transplant recipients. Therefore, a doubling of the dose may be required.
Onychomycosis
Pulse therapy (see table 2).
One course of pulse therapy - 2 caps. Irunin 2 times a day (200 mg 2 times a day), daily, for 1 week.
For the treatment of fungal infections of the nail plates of the hands, two courses are recommended. Three courses are recommended for the treatment of fungal infections of the nail plates of the feet. The interval between courses during which you do not need to take the drug is 3 weeks.
Clinical results will become obvious after the end of treatment, as the nails grow back.
table 2
| Localization of onychomycosis | weeks | ||||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | |
| Foot lesions with or without nail plate lesions | 1st year | Weeks free from taking Irunin | 2nd year | Weeks free from taking Irunin | 3rd year | ||||
| Damage to the nail plates of the hands | 1st year | Weeks free from taking Irunin | 2nd year | — | — | ||||
Or
Continuous treatment: 2 caps. per day (200 mg 1 time per day) for 3 months. The removal of Irunin from the skin and nail tissue is slower than from plasma. Thus, optimal clinical and mycological effects are achieved 2–4 weeks after the end of treatment for skin infections and 6–9 months after the end of treatment for nail infections.
Systemic mycoses (recommended dosages and duration of treatment vary depending on the type of infection - see Table 3)
Table 3
| Indication | Dose | Average duration | Note |
| Aspergillosis | 200 mg 1 time per day | 2–5 months | Increase dose to 200 mg twice daily for invasive or disseminated disease |
| Candidiasis | 100–200 mg 1 time per day | from 3 weeks to 7 months | |
| Cryptococcosis (except meningitis) | 200 mg 1 time per day | from 2 months to 1 year | Maintenance therapy - 200 mg 1 time per day |
| Cryptococcal meningitis | 200 mg 2 times a day | ||
| Histoplasmosis | from 200 mg 1 time per day to 200 mg 2 times per day | 8 months | |
| Sporotrichosis | 100 mg 1 time per day | 3 months | |
| Paracoccidioidomycosis | 100 mg 1 time per day | 6 months | |
| Chromomycosis | 100–200 mg 1 time per day | 6 months | |
| Blastomycosis | from 100 mg 1 time per day to 200 mg 2 times per day | 6 months |
Overdose
Signs of capsule overdose are comparable to dose-dependent adverse events that are observed when taking regular doses of Irunin. Treatment of overdose: there is no specific antidote; Within 1 hour after an overdose, gastric lavage should be performed with soda solution and enterosorbents should be prescribed. Itraconazole is not eliminated by hemodialysis .
The use of vaginal tablets in high doses usually does not cause reactions in the patient that are hazardous to health. In case of accidental ingestion of tablets, it is necessary to perform gastric lavage and prescribe enterosorbents.
Pharmacokinetics
Maximum bioavailability of itraconazole is observed when capsules are taken orally immediately after a heavy meal. Cmax in plasma is achieved within 3-4 hours after oral administration. Elimination from plasma is 2-phase with a final half-life of 1 to 1.5 days. With long-term use, equilibrium concentration is achieved within 1–2 weeks. The concentration of itraconazole in plasma 3-4 hours after taking the drug is 0.4 mcg/ml (100 mg once daily), 1.1 mcg/ml (200 mg once daily) and 2.0 mcg/ml ( 200 mg 2 times a day). Plasma protein binding - 99.8%. The accumulation of the drug in keratin tissues, especially in the skin, is approximately 4 times higher than the accumulation in the blood plasma, and the rate of its elimination depends on the regeneration of the epidermis. In contrast to plasma concentrations, which are undetectable 7 days after cessation of therapy, therapeutic skin concentrations persist for 2-4 weeks after cessation of a 4-week course of treatment. Itraconazole is detected in nail keratin as early as 1 week after the start of treatment and persists for at least 6 months after completion of a 3-month course of therapy. Itraconazole is also detected in sebum and, to a lesser extent, in sweat.
Itraconazole is well distributed in tissues that are susceptible to fungal infections. Concentrations in the lungs, kidneys, liver, bones, stomach, spleen and muscles were 2-3 times higher than the corresponding plasma concentrations. Therapeutic concentrations in vaginal tissues remain for another 2 days after the end of a 3-day course of treatment at a dose of 200 mg per day, and 3 days after the end of a 1-day course of treatment at a dose of 200 mg 2 times a day.
Itraconazole is metabolized by the liver to form a large number of metabolites. One such metabolite is hydroxyitraconazole, which has comparable antifungal activity to itraconazole in vitro. Antifungal concentrations of the drug determined by microbiological methods were approximately 3 times higher than those measured by HPLC. Excretion in feces ranges from 3 to 18% of the dose. Approximately 35% of the dose is excreted as metabolites in the urine within 1 week.
Interaction
When capsules are used together with agents that reduce the acidity of the stomach , the absorption of itraconazole may be weakened.
Agents that stimulate the activity of liver enzymes can reduce the content of itraconazole capsules in the blood.
When taking capsules together with drugs metabolized by the CYP3A isoenzyme (Terfenadine, Astemizole, Vincristine, Cisapride, Midazolam, oral forms of Triazolam, Cyclosporine, indirect anticoagulants, Digoxin, Quinidine, calcium channel blockers) , it is possible that the intensity and duration of their effects may increase.
Irunin capsules 100 mg No. 14
Compound
Active substance: itraconazole 100 mg.
Excipients: hypromellose (hydroxypropyl methylcellulose) - 130.17 mg, methacrylic acid copolymer (eudragit) - 16.26 mg, methyl parahydroxybenzoate (nipagin) - 0.08 mg, propyl parahydroxybenzoate (nipazole) - 0.01 mg, granulated sugar - 163.17 mg, sucrose (sugar) - 54.31 mg . Composition of hard gelatin capsules: titanium dioxide - 1.333%, quinoline yellow dye - 0.9197%, sunset yellow dye - 0.0044%, gelatin - up to 100%.
Pharmacokinetics
Therapeutic concentrations in the skin remain for 2-4 weeks after the end of the 4-week course of treatment. Itraconazole is detected in nail keratin a week after the start of treatment and persists for 6 months after the end of a 3-month course of therapy.
Indications for use
- Dermatomycoses;
- fungal keratitis;
- onychomycosis caused by dermatophytes and/or yeasts and molds;
- systemic mycoses: systemic aspergillosis and candidiasis, cryptococcosis (including cryptococcal meningitis), histoplasmosis, sporotrichosis, paracoccidioidomycosis, blastomycosis and other systemic or tropical mycoses;
- candidomycosis with damage to the skin or mucous membranes, including vulvovaginal candidiasis;
- deep visceral candidiasis; pityriasis versicolor.
Contraindications
- Concomitant oral administration of terfenadine, astemizole, mizolastine, cisapride, dofetilide, quinidine, pimozide, simvastatin, lovastatin, midazolam or triazolam;
- hypersensitivity to itraconazole.
Directions for use and doses
For optimal absorption of the drug, it is necessary to take the drug in capsules immediately after meals. Capsules should be swallowed whole.
For various indications:
- vulvovaginal candidiasis - 200 mg 2 times/day for 1 day or 200 mg 1 time/day for 3 days;
- pityriasis versicolor - 200 mg 1 time/day for 7 days;
- dermatomycosis of smooth skin - 200 mg 1 time/day for 7 days or 100 mg 1 time/day for 15 days;
- fungal keratitis - 200 mg 1 time/day for 21 days;
- lesions of highly keratinized areas, such as the hands and feet, require additional treatment for 15 days at a dose of 100 mg/day;
- oral candidiasis - 100 mg 1 time/day for 15 days.
Therapy for onychomycosis: 200 mg (2 capsules) 2 times a day for 1 week, then 3 weeks interval between courses. In case of damage to the nail plates of the feet with or without damage to the nail plates of the hands - 3 courses of treatment. If only the nail plates of the hands are affected, 2 courses of treatment are required. For onychomycosis, continuous therapy can be used: 200 mg (2 capsules) 1 time/day for 3 months. The elimination of itraconazole from the skin and nail plate is slower than from plasma. Thus, optimal clinical and mycological effects are achieved after 2-4 weeks. after completion of treatment for skin infections and 6-9 months after completion of treatment for nail infections.
Recommended doses for systemic mycoses depending on the type of infection:
- aspergillosis - 200 mg 1 time / day for 2-5 months (increase the dose to 200 mg 2 times / day in the case of invasive or disseminated forms);
- candidiasis - 100-200 mg 1 time / day from 3 weeks to 7 months (increase the dose to 200 mg 2 times / day in the case of invasive or disseminated forms);
- cryptococcosis (except meningitis) - 200 mg 1 time / day from 2 months to 1 year;
- cryptococcal meningitis - 200 mg 2 times a day from 2 months to 1 year;
- histoplasmosis - 200 mg 1 time / day - 2 times / day for 8 months;
- sporotrichosis - 100 mg 1 time/day for 3 months;
- paracoccidioidomycosis - 100 mg 1 time/day for 6 months;
- chromomycosis - 100-200 mg 1 time/day for 6 months;
- blastomycosis - from 100 mg 1 time/day to 200 mg 2 times/day for 6 months.
Storage conditions
At a temperature not higher than 25 °C. Keep out of the reach of children.
Best before date
3 years. Do not use after the expiration date stated on the packaging.
special instructions
- Women of childbearing age taking Irunin must use adequate contraception throughout the course of treatment until the first menstruation after its completion.
- When studying the intravenous dosage form of the drug Itraconazole, a transient asymptomatic decrease in left ventricular ejection fraction was noted, which normalized until the next infusion of the drug.
- Itraconazole has been found to have a negative inotropic effect. Cases of heart failure associated with taking Irunin have been reported. Irunin should not be taken by patients with chronic heart failure or a history of this disease unless the possible benefit significantly outweighs the potential risk.
- Calcium channel blockers may have a negative inotropic effect, which may enhance this effect of itraconazole, and itraconazole may reduce the metabolism of calcium channel blockers. Caution should be used when taking itraconazole and calcium channel blockers concomitantly.
- In patients with renal impairment, the bioavailability of itraconazole may be reduced, which may require dose adjustment.
- With reduced gastric acidity, the absorption of itraconazole is impaired. Patients taking antacid medications (for example, aluminum hydroxide) are recommended to use them no earlier than 2 hours after taking Irunin capsules. Patients with achlorhydria or using H2-histamine blockers or proton pump inhibitors are recommended to take Irunin capsules with acidic drinks.
- In very rare cases, when using Irunin, severe toxic liver damage developed, including cases of acute liver failure with a fatal outcome. This occurred in patients who already had liver disease, as well as in patients receiving other drugs that have hepatotoxic effects. Several such cases occurred in the first month of therapy, and some in the first week of treatment. In this regard, it is recommended to regularly monitor liver function in patients receiving itraconazole therapy.
- Treatment should be discontinued if neuropathy occurs, which may be associated with taking Irunin.
- There is no evidence of cross-hypersensitivity to itraconazole and other azole antifungals. Irunin capsules should be administered with caution to patients with hypersensitivity to other azoles.
- In patients with compromised immunity (AIDS, after organ transplantation, neutropenia), an increase in the dose of Irunin may be required.
Description
Antifungal agent.
Use in children
There is currently insufficient data on the use of itraconazole in children.
Pharmacodynamics
Antifungal agent, triazole derivative. The mechanism of action is associated with the ability to inhibit cytochrome P450-dependent enzymes of sensitive fungi, which leads to disruption of the synthesis of ergosterol in the fungal cell wall. It has a wider spectrum of antifungal action than ketoconazole. Active against Aspergillus spp., Blastomyces dermatitidis, Candida, Coccidioides immitis, Cryptococcus neoformans, Epidermophyton, Microsporum, Trichophyton, Histoplasma capsulatum, Malassezia furfur, Paracoccidioides brasiliensis, Sporothrix schenckii.
Side effects
From the gastrointestinal tract: dyspepsia, nausea, abdominal pain and constipation, reversible increase in the activity of liver enzymes, cholestatic jaundice, hepatitis, anorexia. In very rare cases, when using Irunin, severe toxic liver damage developed, including a case of acute liver failure with a fatal outcome.
From the central nervous system: headache, fatigue, dizziness, peripheral neuropathy.
From the cardiovascular system: congestive heart failure and pulmonary edema.
From other organs and systems: menstrual irregularities, allergic reactions (such as itching, rash, urticaria and angioedema), Stevens-Johnson syndrome, alopecia, hypokalemia, edema, dark urine discoloration, hypercreatininemia.
Use during pregnancy and breastfeeding
During pregnancy, itraconazole is used only for systemic mycoses, when the expected effect of therapy exceeds the potential risk to the fetus.
Women of childbearing potential are advised to use contraception while using itraconazole.
If it is necessary to use it during lactation, the issue of stopping breastfeeding should be decided.
Experimental studies have found that itraconazole has an embryotoxic effect and causes fetal development abnormalities.
Interaction
1. Medicines that affect the metabolism of itraconazole.
The interaction of itraconazole with rifampicin, rifabutin and phenytoin has been studied. The simultaneous use of itraconazole with these drugs, which are powerful inducers of liver enzymes, is not recommended. Interaction studies with other hepatic enzyme inducers, such as carbamazepine, phenobarbital and isoniazid, have not been conducted, but similar results can be expected.
Since itraconazole is primarily metabolized by the enzyme CYP3A4, potent inhibitors of this enzyme may increase the bioavailability of itraconazole. Examples include ritonavir, indinavir, clarithromycin and erythromycin.
2. The effect of itraconazole on the metabolism of other drugs.
Itraconazole may inhibit the metabolism of drugs metabolized by the CYP3A4 enzyme. The result of this may be an increase or prolongation of their action, including side effects.
Drugs that should not be given concomitantly with itraconazole:
- terfenadine, astemizole, mizolastine, cisapride, triazolam and oral midazolam, dofetilide, quinidine, pimozide, HMG-CoA reductase inhibitors such as simvastatin and lovastatin,
- Calcium channel blockers may have a negative inotropic effect, which may enhance the same effect exhibited by itraconazole. Caution should be used when taking itraconazole and calcium channel blockers concomitantly, as the metabolism of calcium channel blockers may be reduced.
Drugs, when prescribed, it is necessary to monitor their plasma concentrations and side effects
In case of simultaneous administration with itraconazole, the dose of these drugs should be reduced, if necessary:
- oral anticoagulants,
- HIV protease inhibitors such as ritonavir, indinavir, saquinavir,
- some anticancer drugs, such as Vinca rosea alkaloids, busulfan, docetaxel, trimetrexate,
- calcium channel blockers broken down by the enzyme CYP3A4, such as verapamil,
- some immunosuppressive drugs: cyclosporine, tacrolimus, sirolimus,
- other drugs: digoxin, carbamazepine, buspirone, alfentanil, alprazolam, brotizolam, rifabutin, methylprednisolone, ebastine, reboxetine.
No interaction was found between itraconazole and zidovudine and fluvastatin.
There was no effect of itraconazole on the metabolism of ethinyl estradiol and norethisterone.
3. Effect on protein binding.
In vitro studies
demonstrated the lack of competition between itraconazole and drugs such as imipramine, propranolol, diazepam, cimetidine, indomethacin, tolbutamide and sulfadimidine in binding to plasma proteins.
Overdose
No data available. During the first hour, perform gastric lavage and, if necessary, prescribe activated charcoal and symptomatic treatment.
Itraconazole is not eliminated by hemodialysis. There is no specific antidote.
Impact on the ability to drive vehicles and operate machinery
Not observed.
special instructions
Special instructions when taking capsules
It is prohibited to use the described medicine in persons with signs of ventricular dysfunction , congestive heart failure, or liver disease. In case of urgent need for kidney disease or liver cirrhosis, treatment with itraconazole is carried out by monitoring its level in the blood.
If the level of liver transaminases in the blood is increased, the drug is used only in cases where the benefit from using the drug is higher than the expected risk of deterioration of the liver.
When using capsules for more than 1 month, regular monitoring of liver function should be carried out.
peripheral neuropathy, appear during therapy, the drug should be discontinued immediately.
Special instructions when taking vaginal tablets
During treatment, it is advised to abstain from sexual intercourse.
To prevent reinfection , therapy should be carried out simultaneously in both sexual partners, and hygiene rules must also be followed.
If an allergy , treatment should be stopped immediately. The drug can be removed by washing the vagina with warm water.
If symptoms of infection persist after treatment, then additional microbiological testing should be performed to clarify the diagnosis.
Use during pregnancy and breastfeeding
Based on the results of preclinical studies and due to the fact that studies on the use of itraconazole in pregnant women have not been conducted, itraconazole should be prescribed to pregnant women only for life-threatening systemic mycoses when the potential benefit to the woman justifies the possible risk to the fetus.
Women of childbearing age taking Irunin must use adequate methods of contraception throughout the entire course of treatment until the onset of the first menstruation after its completion.
Since small amounts of itraconazole are excreted in breast milk, the expected benefit of taking itraconazole must be weighed against the risk to the baby during breastfeeding. If in doubt, do not breastfeed.
Irunin's analogues
Level 4 ATC code matches:
Medoflucon
Vfend
Itracon
Mikosist
Mikomax
Orungal
Mycoflucan
Fluconazole
Sporagal
Orungamin
Flucostat
Difluzol
Rumicosis
Futsis
Vero-Fluconazole
Kandizol
Kanditral
Itraconazole
Diflazon
Itrazol (capsules), Itraconazole , Itramikol , Canditral (capsules), Orungal (capsules, solution), Orungamin , Rumikoz (capsules), Teknazol , Zalain (suppositories), Vagisept (suppositories), Fluconazole , Livarol (suppositories), Mikosist (capsules) , solution), Nizoral (tablets), Clotrimazole (ointment, cream).
Irunin is a relatively inexpensive drug; analogues are usually more expensive.
Irunin price, where to buy
The price of Irunin in vaginal tablets No. 10 in Russia is 280-315 rubles. The price of Irunin candles in Ukraine reaches 400 hryvnia for package No. 10.
The price of Irunin for nail fungus (capsule No. 14) in Russia ranges from 650-750 rubles. Buying capsules in this form of release reaches 735 hryvnia.
- Online pharmacies in RussiaRussia
ZdravCity
- Irunin capsules 100 mg 14 pcs. JSC Veropharm
RUB 892 order - Irunin capsules 100 mg 6 pcs. JSC Veropharm
RUR 497 order
- Irunin capsules 100 mg 28 pcs JSC Veropharm
RUB 1,516 order