What is Longidaza
Combined pharmaceutical preparation for local use and parenteral administration. It has an immunomodulatory and anti-inflammatory effect. The product contains several active compounds: azoximer bromide and the enzyme hyaluronidase.
The medicine is produced in the form of:
- lyophilisate: porous white substrate for injection, available in ampoules, 15 mg of the drug contains 1500 active IU and stabilizers;
- fat-based suppositories that are administered rectally or intravaginally: in cell blisters of 5 or 10 copies.
How does Longidaza work?
The active components of the drug promote the resorption of edema and hematomas, increase the permeability of soft tissues, reduce the concentration of fibrin in areas of damage, slow down scarring, help maintain the elasticity of connective fibers, and accelerate the production of collagen.
Longidaza strengthens the body's defenses, inhibits inflammatory reactions, and prevents the growth of adhesive structures. In complex treatment, the drug increases the effectiveness of other medications, including painkillers. The product is not toxic and does not accumulate or be deposited in the body. After administration, Longidaza is distributed in tissues, gradually undergoes hydrolysis, and is completely eliminated from the body within 5 days.
Pharmacokinetics
An experimental study of the pharmacokinetics of suppositories with an enzyme carrier labeled with tritium made it possible to establish that when administered rectally, the drug is characterized by a high rate of distribution in the body, is well absorbed into the systemic circulation and reaches Cmax in the blood after 1 hour. The half-life of distribution is about 0.5 hours, period elimination half-life is from 42 to 84 hours. It is excreted mainly by the kidneys.
The drug penetrates into all organs and tissues, incl. passes through the BBB and the blood-ophthalmic barrier. The absence of tissue cumulation was established.
The bioavailability of Longidase® rectal suppositories is at least 70%.
For what pathologies is Longidaza necessary?
The medication is prescribed to prevent or eliminate hyperplasia of connective tissue areas:
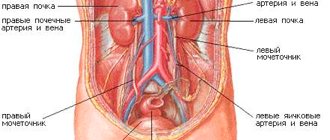
- when adhesions form in the pelvic area;
- endometritis;
- infertility;
- with interstitial type of cystitis;
- with prostatitis;
- to slow down the formation of keloid scars, prevent tissue hypertrophy after mechanical damage;
- with limited scleroderma;
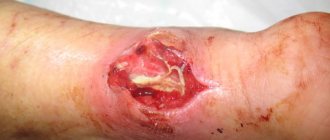
- for healing old wounds and fistulas;
- for pneumosclerosis, alveolitis with a risk of developing pulmonary fibrosis;
- for impaired joint mobility, arthritis and spondyloarthritis;
- severe hematomas.
Nosological classification (ICD-10)
- A16.9 Respiratory tuberculosis of unspecified localization without mention of bacteriological or histological confirmation
- J63.4 Siderosis
- J84.1 Other interstitial lung diseases with mention of fibrosis
- J84.8 Other specified interstitial pulmonary diseases
- L94.0 Localized scleroderma [morphea]
- N13.5 Kink and stricture of the ureter without hydronephrosis
- N30.1 Interstitial cystitis (chronic)
- N35 Urethral stricture
- N40 Prostatic hyperplasia
- N41.1 Chronic prostatitis
- N48.8 Other specified diseases of the penis
- N71 Inflammatory diseases of the uterus, except the cervix
- N85.6 Intrauterine synechiae
- N97.1 Female infertility of tubal origin
- N99.1 Postoperative urethral stricture
- N99.4 Postoperative adhesions in the pelvis
- T14.1 Open wound of unspecified body area
- Z100* CLASS XXII Surgical practice
When is Longidaza contraindicated?
The use of the drug is prohibited:
- in case of individual intolerance to components;
- internal bleeding;
- blood impurities in mucous sputum;
- in acute infectious processes;
- severe renal impairment;
- recent hemorrhages in the eyeball area;
- after hemorrhagic stroke.
Patients under 18 years of age should avoid using Longidase.
Pharmacodynamics
Longidase® has hyaluronidase (enzymatic proteolytic) activity of prolonged action, chelating, antioxidant, immunomodulatory and moderate anti-inflammatory properties.
Prolongation of the action of the enzyme is achieved by covalent binding of it to a physiologically active high-molecular carrier (activated derivative of poly-1,4-ethylenepiperazine N-oxide, an analogue of the drug Polyoxidonium®), which has its own pharmacological activity. Longidase® exhibits antifibrotic properties, weakens the course of the acute phase of inflammation, regulates (increases or decreases depending on the initial level) the synthesis of inflammatory mediators (IL-1 and tumor necrosis factor-alpha), increases the humoral immune response and the body's resistance to infection.
The pronounced antifibrotic properties of Longidase® are ensured by the conjugation of hyaluronidase with a carrier, which significantly increases the resistance of the enzyme to denaturing influences and the action of inhibitors: the enzymatic activity of Longidase® is maintained when heated to 37 °C for 20 days, while native hyaluronidase loses under the same conditions your activity during the day. The Longidaza® preparation ensures the simultaneous local presence of the proteolytic enzyme hyaluronidase and a carrier capable of binding matrix components released during hydrolysis, enzyme inhibitors and stimulators of collagen synthesis (including iron, copper ions, heparin). Thanks to these properties, Longidaza® has not only the ability to depolymerize the connective tissue matrix in fibrogranulomatous formations, but also suppress the reverse regulatory reaction aimed at the synthesis of connective tissue components.
The specific substrate of testicular hyaluronidase is glycosaminoglycans (hyaluronic acid, chondroitin, chondroitin-4-sulfate, chondroitin-6-sulfate), which form the basis of the connective tissue matrix. As a result of depolymerization (breaking the bond between C1 acetylglucosamine and C4 glucuronic or induronic acids), glycosaminoglycans change their basic properties: viscosity decreases, the ability to bind water and metal ions decreases, the permeability of tissue barriers temporarily increases, fluid movement in the intercellular space is facilitated, and the elasticity of connective tissue increases. , which manifests itself in a decrease in tissue swelling, flattening of scars, an increase in the range of motion of joints, a decrease in contractures and prevention of their formation, and a decrease in adhesions.
Biochemical, immunological, histological and electron microscopic studies have proven that Longidaza® does not damage normal connective tissue, but causes destruction of connective tissue altered in composition and structure in the area of fibrosis.
Longidaza® does not have mutagenic, embryotoxic, teratogenic or carcinogenic effects.
The drug was well tolerated by patients; no local or general allergic reactions were noted.
The use of Longidase® in therapeutic doses during or after surgical treatment does not cause worsening of the postoperative period or progression of the infectious process; does not slow down bone tissue recovery.
How to give Longidaza injections
Before placing the suppository into the intestines, you must go to the toilet, and if necessary, do an enema. The drug is administered in a lying position, 6–10 cm deep into the rectum. The optimal time is before a night's rest.
Vaginal administration is carried out in the same way: after hygienic procedures, in a lying position. Within 1.5–2 hours after placing the medicine inside, vigorous activity and vertical body position are not recommended. It is advisable to use candles while relaxing or before bed.
The course of therapy with suppositories depends on the type of disease:
- for gynecological pathologies: 1 suppository every three days, a total of 10 procedures;
- for skin inflammations or wounds: rectally, a suppository every 2 days, 15 pieces in total;
- for surgical lesions: every 4 days rectally, 10 procedures per course;
- in pulmonology: rectally every 3 or 5 days, course duration - from 10 to 20 suppositories.
The drug is used with caution in cases of kidney dysfunction. In this case, a longer interval between administrations of suppositories is required - about 7 days.
The doctor may suggest a treatment regimen that differs from that recommended in the instructions.
Longidaza, 3000 IU, lyophilisate for the preparation of solution for injection, 5 pcs.
PC
(near the site of the lesion or under scar tissue) or
intramuscularly
at a dose of 3000 IU in a course of 5 to 25 injections (depending on the disease) with an interval between injections of 3 to 10 days.
Methods of application are selected by the doctor depending on the diagnosis, severity of the disease, clinical course, and age of the patient.
If necessary, a repeat course is recommended after 2–3 months.
In the case of treatment of diseases accompanied by a severe chronic productive process in the connective tissue, after a standard course, long-term maintenance therapy with Longidase® 3000 IU is recommended with breaks between injections of 10–14 days.
To increase bioavailability
For medicinal and diagnostic agents, a dose of 1500 IU is recommended with preliminary (10–15 min) intramuscular or subcutaneous administration to the same site as the main drug.
Breeding
1. The contents of the ampoule or bottle of Longidaza® 3000 IU are dissolved in 1–2 ml of procaine solution (0.25 or 0.5%). In case of intolerance to procaine, Longidaza® is dissolved in the same volume of 0.9% sodium chloride solution for injection or water for injection.
2. When used to increase bioavailability, the contents of an ampoule or vial of Longidaza® 3000 IU are dissolved in 2 ml, and with a dosage of 1500 IU - in 1 ml of sodium chloride solution 0.9% for injection.
The solvent must be introduced into the vial or ampoule slowly, wait for 2–3 minutes, and carefully mix without shaking so as not to foam the protein.
The prepared solution for parenteral administration cannot be stored. Do not administer intravenously!
Recommended prevention and treatment regimens
For the prevention of adhesive disease and severe scarring after surgical interventions on the abdominal and pelvic organs - IM at a dosage of 3000 IU once every 3 days, in a course of 5 injections. If necessary, the use of Longidaza® can be continued with a total course of up to 10 injections when administered once every 5 days.
For treatment
in gynecology:
- adhesions in the pelvis during inflammatory diseases of the internal genital organs - 3000 IU intramuscularly once every 3-5 days, course - 10-15 injections;
— tubo-peritoneal infertility — 3000 IU intramuscularly for a total course of up to 15 injections: the first 5 injections — once every 3 days, then — once every 5 days;
in urology:
— chronic prostatitis — 3000 IU intramuscularly once every 5 days, course — 10–15 injections;
- interstitial cystitis - 3000 IU intramuscularly once every 5 days, course - up to 10 injections;
in surgery:
- adhesive disease after surgical interventions on the abdominal organs - IM at a dosage of 3000 IU once every 3-5 days, course - from 10 to 15 injections;
- long-term non-healing wounds - IM at a dosage of 3000 IU once every 5 days, course - 5-10 injections;
in dermatovenerology, cosmetology:
— limited scleroderma — 3000–4500 IU intramuscularly, once every 3–5 days, in a course of up to 20 injections. The dosage and course are selected individually, depending on the clinical course, stage, location of the disease and the individual characteristics of the patient;
- keloid, hypertrophic and developing scars after pyoderma, burns, operations, injuries - intra-scar or subcutaneous (near the site of the lesion) administration in a dosage of 3000-4500 IU, 1 time in 3 days, in a course of up to 15 injections. The volume of dilution of Longidaza® is selected by the doctor depending on the number of injection points. If necessary, the course can be continued according to the scheme once every 5 days up to 25 injections. Depending on the area of skin damage and how long ago the scar formed, alternating subcutaneous and intramuscular administration is possible once every 5 days at a dosage of 3000 IU, in a course of up to 20 injections;
in pulmonology and phthisiology:
- pneumosclerosis - 3000 IU intramuscularly once every 5 days, course - 10 injections;
— fibrosing alveolitis — intramuscularly at a dosage of 3000 IU once every 5 days, a course of 15 injections, then maintenance therapy — once every 10 days for a total course of up to 25 injections;
— tuberculosis — intramuscularly at a dosage of 3000 IU once every 5 days, in a course of up to 25 injections; depending on the clinical picture and severity of the disease, long-term therapy is possible (from 6 months to 1 year at a dosage of 3000 IU once every 10 days);
in orthopedics:
- joint contracture - subcutaneous injection near the site of the lesion at a dosage of 3000 IU once every 3 days, course - from 5 to 15 injections;
- arthrosis, ankylosing spondylitis - subcutaneously near the site of the lesion at a dosage of 3000 IU once every 3 days, a course of up to 15 injections, if necessary, treatment can be continued with injections once every 5 days. The duration of maintenance therapy is selected by the doctor depending on the severity of the disease;
— hematomas — subcutaneously near the site of the lesion at a dosage of 3000 IU once every 3 days for a course of up to 5 injections;
to increase bioavailability:
when administered subcutaneously or intramuscularly with diagnostic or medicinal drugs (including antibiotics, chemotherapy drugs, anesthetics). Longidaza® is pre-administered 10–15 minutes in advance at a dosage of 1500 IU in the same way and in the same place as the main drug.