Compound
- 1 tablet of Zovirax contains 200 mg of acyclovir . Additional components: povidone K30, lactose monohydrate, sodium starch glycolate, microcrystalline cellulose, magnesium stearate.
- 1 gram of Zovirax cream contains 50 mg of acyclovir . Auxiliary components: dimethicone, white paraffin, propylene glycol, cetostearyl alcohol, poloxamer 407, liquid paraffin, sodium lauryl sulfate, macrogol stearate, glycerol monostearate, water.
- 1 gram of Zovirax ointment contains 30 mg of acyclovir . Auxiliary components: white Vaseline.
- 1 bottle of lyophilisate for preparing Zovirax injections contains 250 mg of acyclovir . Auxiliary components: sodium hydroxide.
Release form
White, biconvex, round tablets, engraved with “GXCL3”.
- 5 tablets in a contour pack, 5 packs in a paper pack.
White homogeneous cream 5% for external use.
- 2 grams of cream in a plastic bottle with a dispenser; one bottle in a paper pack.
- 2, 5 or 10 grams of cream in an aluminum tube, 1 tube in a paper pack.
White, translucent, homogeneous, oily ointment, with a slight odor, containing no lumps, grains or foreign inclusions.
- 4.5 grams of cream in a tube with a plastic nozzle, 1 tube in a paper pack.
The lyophilisate for the preparation of injections is a white powder (hygroscopic or in the form of a sintered mass).
- 250 mg powder in a glass bottle, 5 bottles in a plastic tray; one tray in a cardboard box.
Description of the dosage form
Tablets: blue, flat, shield-shaped (irregular polygonal) tablets; On one side there is the inscription “ZOVIRAX” and on the other there is a triangle. Minor inclusions may be visible on the surface of the tablet.
Powder for the preparation of solution for injection: sintered mass (porous cake) or lyophilized powder of white or almost white color. The drug is hygroscopic.
Eye ointment: soft, homogeneous, white or almost white, translucent oily mass with a weak characteristic odor, free of grains, lumps and foreign particles.
Pharmacodynamics and pharmacokinetics
Pharmacodynamics
Antiviral drug , an artificial analogue of a purine-type nucleoside , which has the ability to inhibit the replication of herpes simplex viruses (HSV) of all types, Varicella zoster, cytomegalovirus and Epstein-Barr . Acyclovir has the most pronounced antiviral properties against the herpes virus type 1 .
The effect of the drug on viruses is highly selective. Thymidine kinase of cells infected with these viruses converts acyclovir into monophosphate , then successively into diphosphate and triphosphate under the influence of cell enzymes. The inclusion of triphosphate in the DNA and the subsequent termination of this chain blocks the copying of viral DNA .
In patients with severe immunodeficiency , prolonged or repeated courses of treatment with acyclovir can lead to the emergence of strains resistant to the drug. Many strains with reduced sensitivity to Zovirax had low concentrations of viral thymidine kinase .
Pharmacokinetics of Zovirax tablets and injections
When taken orally, the active substance is only partially absorbed from the intestine. The content in the cerebrospinal fluid is approximately half of its plasma concentration. Bounds to blood proteins to a small extent (10-33%).
The main metabolite is 9-carboxymethoxy-methylguanine . The half-life is 2.7-3.3 hours. Most of the drug is excreted by the kidneys in unchanged form. It is excreted not only through glomerular filtration, but also through tubular secretion.
In individuals with chronic renal failure, the half-life of acyclovir approaches 19.5 hours. In elderly patients, the clearance of acyclovir decreases with age, but the half-life changes slightly.
Pharmacokinetics of Zovirax ointment
After applying the eye ointment, the active substance is quickly absorbed by the periocular tissues and the corneal epithelium, after which the concentration of the drug necessary to suppress the virus is created in the fluid inside the eye. Acyclovir with this method of administration is determined in the urine in a very low concentration, which has no clinical significance.
Pharmacokinetics of Zovirax cream
With repeated use of acyclovir , systemic absorption is minimal.
Pharmacological properties
Zovirax eye ointment is an antiviral drug that is highly active against Varicella zoster and Herpes simplex viruses (types 1, 2).
Penetrating into infected cells, acyclovir is phosphorylated under the influence of viral thymidine kinase to acyclovir triphosphate. Acyclovir triphosphate inhibits viral DNA synthesis but does not damage host cells.
After instillation of the ointment into the eye, acyclovir is absorbed by the corneal epithelium, as well as by the periocular tissues, after which its concentration necessary to neutralize the virus is created in the aqueous humor.
Indications for use
Indications for use of the tablet form of the drug:
- therapy for infectious lesions of Varicella zoster ( herpes zoster and chickenpox virus );
- therapy for infectious lesions of the skin and mucous membranes of HSV of all types, including genital herpes in primary and recurrent forms ;
- HSV infections of all types, in persons with normal immunity or immunodeficiency ;
- therapy of patients with severe forms of immunodeficiency , mainly with HIV infection (with CD4+ less than 200 cells/μl with early manifestations of HIV infection and AIDS ) or after bone marrow transplantation.
Indications for use of Zovirax ointment:
- keratitis caused by HSV of all types.
Indications for use of Zovirax cream:
- infectious lesions of HSV of all types of skin and mucous membranes, including herpes lips.
Indications for the use of Zovirax lyophilisate for the preparation of injections:
- HSV infections of all types;
- prevention of infectious lesions of HSV of all types of skin and mucous membranes in persons with immunodeficiency ;
- therapy for infectious lesions of Varicella zoster;
- HSV infections of all types in newborns;
- prevention of cytomegalovirus infection after bone marrow transplantation.
Zovirax for colds on the lips, cream 5% 5g
A country
Great Britain, United Kingdom
Country of origin may vary depending on product batch. Please check with the operator for detailed information when confirming your order.
Active substance
Acyclovir
Description
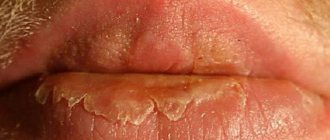
Zovirax cream is used to treat cold sores (herpes).
To achieve maximum effect, it is necessary to use Zovirax cream at the first signs of the disease: - Burning - Itching - Tingling - Sensation of tension - Redness* Zovirax cream is convenient to take with you thanks to its compact packaging (5g). * Instructions for medical use, RU No. P N014304/01 dated December 25, 2007
Compound
Active substance: acyclovir 5.00 g. Excipients: poloxamer 407 1.00 g, cetostearyl alcohol 6.75 g, white soft paraffin 11.50 g, sodium lauryl sulfate 0.75 g, liquid paraffin 5.00 g, glycerol monostearate 0.75 g, macrogol stearate 0.75 g, propylene glycol 40.00 g, dimethicone 1.00 g, purified water up to 100.00 g.
Product description
A homogeneous cream of white or almost white color, without small, large or foreign particles, without signs of separation.
pharmachologic effect
Acyclovir, an antiviral drug, is highly active in vitro against Herpes simplex virus (HSV) types 1 and 2. Low toxicity to mammalian host cells. After entering cells infected with the herpes virus, acyclovir is phosphorylated to the active compound acyclovir triphosphate. The first step of this process depends on the presence of the HSV-encoded thymidine kinase enzyme. Acyclovir triphosphate acts as an inhibitor and substrate of herpes-specific DNA polymerase, preventing further synthesis of viral DNA, without affecting normal cellular processes.
Pharmacokinetics
Pharmacological studies have shown minimal systemic absorption with repeated use of acyclovir cream.
Indications for use
Treatment of viral infections caused by the Herpes simplex virus, lips and facial skin (recurrent herpes of the lips).
Contraindications
Hypersensitivity to acyclovir, valacyclovir, propylene glycol or other components of the drug.
Use during pregnancy and lactation
Pregnancy: Use is only possible in cases where the expected benefit outweighs the potential and unknown risk, however, systemic exposure with topical application of the cream is very low. As a result of post-registration experience with the use of acyclovir, data were obtained containing information on the results of pregnancy in women receiving Zovirax in any dosage form. The data do not demonstrate an increase in the incidence of congenital malformations among patients treated with acyclovir compared with the general population, and no birth defects have been reported that are unique or have a consistent pattern of features suggestive of a common cause. Before use, if you are pregnant, or think you might be pregnant, or are planning a pregnancy, or while breastfeeding, you should consult your doctor. Lactation: Limited data show that with regular use the drug passes into breast milk. However, the dosage that a breastfed infant receives will be negligible.
Directions for use and doses
For external use only. Use the drug only according to the indications, method of administration and in the doses indicated in the instructions. Adults and children: The drug is recommended to be applied 5 times a day, approximately every 4 hours, with the exception of night time, to the affected and adjacent areas of the skin. It is important to start treatment as early as possible, preferably when the first signs and symptoms appear (in the prodromal period or with redness). Treatment can also begin at later stages (papule or blister). The duration of treatment is at least 4 days. If there is no healing, treatment can be continued for up to 10 days. If symptoms persist for more than 10 days, you should consult a doctor. To prevent the condition from worsening and to prevent the spread of infection, you must wash your hands before and after applying the drug, do not rub or touch the affected areas of the skin with a towel.
Side effect
Side effects are classified by organ system and frequency. The frequency of side effects is determined as follows: very often ≥ 1/10, often ≥ 1/100 and From the skin and subcutaneous tissues Uncommon - short-term burning, tingling in the areas where the drug was applied, slight dryness or peeling, itching; Rarely – redness; contact dermatitis after application, more often associated with a reaction to excipients than to acyclovir. Immune system disorders Very rare - immediate hypersensitivity reactions, including angioedema, urticaria. If you experience side effects listed in the instructions or they get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Overdose
Due to minimal systemic exposure, no adverse effects are expected to occur when taken orally or when applied topically to the entire contents of the consumer package of Zovirax Cream. You should consult a doctor if you suspect an overdose.
Interaction with other drugs
When used externally, no interactions with other drugs have been identified.
special instructions
Zovirax cream for external use should be used exclusively for herpes of the skin of the lips and face. It is not recommended to use on the mucous membranes of the mouth, eyes and vagina. Do not use for the treatment of genital herpes. Avoid getting the cream into your eyes. In case of severe manifestations of recurrent herpes, it is recommended to consult a doctor. Patients with herpes labialis should avoid transmitting the virus, especially if active lesions are present. Patients with immunodeficiency conditions are not recommended to use Zovirax cream for external use. Such patients should follow the doctor's recommendations when treating any infectious diseases. Patients with renal and hepatic insufficiency: Despite the fact that acyclovir is mainly excreted by the kidneys, the systematic absorption of acyclovir after external use is negligible. Accordingly, there is no need to change the dose in patients with renal or hepatic impairment
Release form
Cream for external use, 5%. 5 g of the drug in an aluminum tube with a screw-on plastic cap or 2 g in a plastic bottle with a dosing device, sealed with a plastic cap. An aluminum tube or plastic bottle is placed in a cardboard box along with instructions for medical use. Secondary packaging is allowed to have a first-opening control.
Storage conditions
Store at a temperature not exceeding 25 C. Do not freeze. Keep out of the reach of children
Best before date
In an aluminum tube - 3 years, in a plastic bottle with a dispensing device - 2 years. Do not use after the expiration date stated on the packaging.
Contraindications
- Allergy to acyclovir or valacyclovir or components of the drug.
- Contraindications for intravenous administration of the drug: renal failure, dehydration , reactions to intravenous administration of cytotoxic drugs (including in the past), neurological disorders, pregnancy.
- Contraindications for oral administration of the drug: renal failure, dehydration .
Side effects
Side effects when using tablets and lyophilisate
- Digestive reactions: vomiting, nausea, diarrhea and abdominal (if taken orally).
- Hematopoietic reactions: leukopenia, anemia and thrombocytopenia .
- Hypersensitivity reactions: rash, fever , itching, shortness of breath, angioedema , anaphylaxis , urticaria , photosensitivity, severe inflammatory reactions at the site of parenteral administration.
- Reactions from the kidneys: increased concentrations of creatinine and urea in the blood. To avoid such phenomena, instead of a single-stage intravenous injection, it is necessary to prescribe a slower administration over 1 hour. Renal failure caused by intravenous administration of Zovirax is usually relieved by rehydration or reducing the dosage of the drug.
- Liver reactions: temporary increase in bilirubin and liver enzymes, hepatitis and jaundice (rarely with parenteral administration).
- Reactions from nervous activity: psychosis , confusion, tremor , nervous agitation, drowsiness, convulsions , hallucinations , coma , headache (if taken orally).
- Other reactions: fatigue, hair loss.
Side effects when using eye ointment
- Immune reactions: hypersensitivity reactions up to angioedema .
- Visual reactions: punctate keratopathy (passes without consequences and does not require stopping treatment), mild temporary burning sensation, conjunctivitis , blepharitis.
Side effects when using the cream
- Local reactions: temporary itching, redness, burning, peeling, tingling in the area of application.
- Allergic reactions: dermatitis , Quincke's edema .
Instructions for use of Zovirax (Method and dosage)
Zovirax tablets, instructions for use
Zovirax tablets are taken orally with 200 ml of water during meals.
When treating infectious lesions of HSV , 200 mg of the drug is prescribed every 4 hours, five times a day. The standard course of treatment is 5 days, but for severe infections it is allowed to be extended. In the presence of severe immunodeficiency or in cases of intestinal absorption disorders, the dosage of Zovirax can be increased to 400 mg while maintaining the same frequency of administration. It is recommended to begin treatment as quickly as possible after the infection develops; in case of relapses, it is recommended to take the medicine when the first symptoms appear.
To prevent relapses of HSV infections in persons with normal immunity, 200 mg of the drug is recommended four times a day at regular intervals. For many, a more convenient dosage schedule is suitable - 400 mg twice a day. In some cases, low dosages of the drug are effective - 200 mg 3-2 times a day. In some patients, the progression of infection may be interrupted when taking a total dose of 800 mg per day.
Zovirax therapy should be stopped periodically for 6–12 months in order to detect changes in the course of the infection.
HSV infections in people with immunodeficiency , 200 mg of the drug is prescribed four times a day. In the presence of severe immunodeficiency or in case of impaired absorption from the intestine, the dosage can be increased to five times 400 mg of the drug per day. The time of preventive treatment is determined by the duration of the infectious period.
When treating herpes zoster and chickenpox, a five-time dose of 800 mg of the drug per day is prescribed (except during night sleep). The duration of such treatment is one week. The drug should be prescribed as quickly as possible after the onset of infection, because in this case the therapy is most effective.
For the treatment of patients with severe forms of immunodeficiency , four doses of 800 mg of the drug per day are prescribed at regular intervals. Persons who have undergone a bone marrow transplant are usually advised to undergo a month's course of parenteral therapy with Zovirax before taking Zovirax tablets. The maximum duration of treatment after bone marrow transplantation was 6 months. In patients with advanced clinical HIV infection, the duration of treatment was 1 year.
For patients with severe forms of renal failure, the dosage of Zovirax is recommended to be reduced to 200 mg twice a day.
In the treatment of herpes zoster and chickenpox , as well as in the treatment of persons with severe immunodeficiency , standard dosages are:
- severe renal failure - 800 mg twice a day;
- moderate form of renal failure - 800 mg three times a day.
Zovirax eye ointment, instructions for use
Zovirax eye ointment is placed into the conjunctival sac in a 10 mm strip up to 5 times a day. It is recommended to continue treatment after recovery for at least another 3 days.
Zovirax cream, instructions for use
Zovirax cream is applied with a cotton swab or pre-washed hands to avoid reinfection of the affected areas.
A small amount of the medicine is applied to the affected and adjacent areas of the skin and mucous membranes up to 5 times a day.
The duration of treatment is usually 4 days. If there is no healing, it can be extended up to 10 days. If symptoms of the disease persist after 10 days of treatment, you should consult your doctor.
Lyophilisate Zovirax for the preparation of injections, instructions for use
The prepared solution is administered intravenously. In obese individuals, the same dosages are used as in individuals of normal weight.
For the treatment of infectious lesions of HSV and herpes zoster virus , the drug is administered intravenously three times a day at a dose of 5 mg/kg body weight.
For the treatment of infectious lesions with the herpes zoster virus and herpetic encephalitis in people with immunodeficiency , the drug is administered intravenously three times a day at a dose of 10 mg/kg body weight.
To prevent cytomegalovirus infection during bone marrow transplantation, the drug is administered intravenously three times a day at a dose of 500 mg/m2 body area. Therapy begins 5 days before transplantation and continues until 30 days after transplantation.
Pay special attention to reducing Zovirax dosages in elderly people with reduced creatinine clearance .
In people with renal failure, intravenous administration of Zovirax should be prescribed with caution. The dosage will be changed depending on the severity of the deficiency.
The course of therapy with Zovirax in the form of intravenous infusions is usually 5 days, but can be adjusted depending on the patient’s condition and response to therapy. The duration of preventive treatment is determined by the duration of the infectious-dangerous period.
Preparation of solution and method of administration
Zovirax must be administered intravenously, slowly, over 1 hour.
To prepare a solution of the drug with a concentration of the active substance of 25 mg/ml, you need to add 10 ml of water or saline solution into the ampoule with powder and shake gently until the contents are completely dissolved.
Another method of infusion administration is possible: the prepared solution is diluted further to a concentration of 5 mg/ml. To do this, add the prepared solution to one of the infusion solutions and shake to completely mix the solutions. For adults, it is recommended to use infusion solutions in 100 ml bags, despite obtaining a dilution of acyclovir of less than 0.5%. Zovirax for intravenous administration is compatible with the following solutions and remains stable for 12 hours at a temperature of 15-24 °C:
- 0.18% sodium chloride and 4% glucose ;
- 0.45% sodium chloride and 2.5% glucose ;
- 0.45% or 0.9% sodium chloride ;
- Hartmann's solution.
Pharmacokinetics
After oral administration, acyclovir is only partially absorbed from the intestine. When taking 200 mg of acyclovir every 4 hours, the mean maximum steady-state plasma concentration (Cssmax) was 3.1 µmol (0.7 µg/ml), and the mean steady-state minimum plasma concentration (Cssmin) was 1.8 µmol (0.4 µg/ml). When taking 400 and 800 mg of acyclovir every 4 hours, Cssmax was 5.3 µmol (1.2 µg/ml) and 8 µmol (1.8 µg/ml), respectively, and Cssmin was 2.7 µmol (0.6 µg/ml ) and 4 µmol (0.9 µg/ml), respectively.
After intravenous administration of acyclovir to adults, the mean Cmax values 1 hour after infusion at doses of 2.5 mg/kg, 5 mg/kg, 10 mg/kg and 15 mg/kg were 22.7 µmol (5.1 µg/ml ); 43.6 µmol (9.8 µg/ml); 92 µmol (20.7 µg/ml) and 105 µmol (23.6 µg/ml), respectively. Cmin 7 hours after infusion was respectively equal to 2.2 µmol (0.5 µg/ml); 3.1 µmol (0.7 µg/ml); 10.2 µmol (2.3 µg/ml) and 8.8 µmol (2.0 µg/ml). In children over 1 year of age, similar Cmax and Cmin were observed when administered at a dose of 250 mg/m2 instead of 5 mg/kg (adult dose) and at a dose of 500 mg/m2 instead of 10 mg/kg (adult dose). In neonates (0 to 3 months) who received acyclovir as an infusion over 1 hour every 8 hours, Cmax was 61.2 µmol (13.8 µg/ml) and Cmin was 10.1 µmol (2.3 µg/ml). Their T1/2 was 3.8 hours.
T1/2 in adults is 2.5–3.3 hours. Most of the drug is excreted unchanged by the kidneys. The renal clearance of acyclovir significantly exceeds the clearance of creatinine, which indicates that acyclovir is eliminated through not only glomerular filtration, but also tubular secretion. The main metabolite of acyclovir is 9-carboxymethoxy-methylguanine, which accounts for about 10–15% of the administered dose in the urine. When acyclovir was administered 1 hour after taking 1 g of probenecid, the T1/2 of acyclovir and the area under the plasma concentration-time curve increased by 18 and 40%, respectively.
In elderly people, the clearance of acyclovir decreases with age in parallel with a decrease in creatinine clearance, but the T1/2 of acyclovir changes slightly.
In patients with chronic renal failure, T1/2 of acyclovir averaged 19.5 hours, and during hemodialysis, the average T1/2 of acyclovir was 5.7 hours, and the concentration of acyclovir in plasma decreased by approximately 60%.
The concentration of acyclovir in the cerebrospinal fluid is approximately 50% of its plasma concentration. Acyclovir binds to plasma proteins to a small extent (9–33%), so drug interactions due to displacement from protein binding sites are unlikely.
When acyclovir and zidovudine were administered simultaneously to HIV-infected patients, the pharmacokinetic characteristics of both drugs remained virtually unchanged.
After applying ophthalmic ointment, acyclovir is quickly absorbed by the corneal epithelium and periocular tissues, resulting in the concentration of the drug necessary to suppress the virus in the intraocular fluid. After using Zovirax ophthalmic ointment, acyclovir is detected only in the urine, and in small quantities.
Overdose
There is no information about an overdose of the drug in the form of a cream or eye ointment.
Overdose of pills
With a single random oral dose of up to 20 grams, no undesirable effects are recorded.
Signs of overdose: nausea, vomiting, headache, shortness of breath, confusion, renal dysfunction, diarrhea , convulsions , lethargy , coma .
Careful observation is necessary in order to timely identify possible signs of intoxication. of hemodialysis cannot be ruled out .
Overdose with solution
Signs of overdose: increased levels of urea nitrogen and creatinine in the blood, hallucinations, renal failure , confusion, convulsions , agitation, coma .
Hemodialysis is recommended , which significantly enhances the evacuation of acyclovir from the body and is the optimal method of treatment for overdose of injectable forms of Zovirax.
Use during pregnancy and breastfeeding
An analysis of acyclovir treatment of women during pregnancy did not reveal an increase in the number of birth defects in their children compared to the general population. However, caution should be exercised when prescribing Zovirax to women during pregnancy and assess the expected benefit to the mother and the possible risk to the fetus.
After taking Zovirax orally at a dose of 200 mg 5 times a day, acyclovir was detected in breast milk in concentrations ranging from 0.6 to 4.1 of plasma concentrations. At such concentrations in breast milk, breastfed children can receive acyclovir at a dose of up to 0.3 mg/kg/day. Given this fact, caution should be exercised when prescribing Zovirax to nursing women.
Interaction
No significant drug interactions were detected when using the drug.
Acyclovir is excreted unchanged into the urine due to tubular secretion . All drugs with a similar method of elimination can increase the concentration of acyclovir in the blood.
Caution should be exercised in combining intravenous administration of Zovirax with drugs that interfere with kidney function ( Ciclosporin , Tacrolimus and others).
special instructions
Patients taking the drug orally in large doses should drink sufficient fluid.
It is possible that a burning sensation may occur immediately after applying the eye ointment, which usually goes away spontaneously.
During drug therapy, wearing contact lenses is prohibited.
In order for the therapeutic effect to be maximum, it is necessary to start using the drug already at the initial symptoms of the disease (tingling, itching, burning, redness).
If you have severe symptoms of herpes , you should definitely consult a doctor.
When treating genital herpes , it is advised to abstain from sexual intercourse or use condoms, since the use of acyclovir does not protect against contracting the disease through sexual contact.
It is not recommended to apply the cream to the mucous membranes of the mouth and eyes due to the fact that local inflammation may occur.
Patients with immunodeficiency should adhere to the recommendations of the attending physician when treating any infectious lesions.
In persons with herpetic encephalitis , taking Zovirax in large dosages, it is necessary to constantly monitor kidney function.
The finished solution of the drug has a pH of 11, so it is prohibited for oral use.
Precautionary measures
In case of renal failure, Zovirax doses should be adjusted according to its degree to prevent the accumulation of acyclovir in the body.
In patients receiving IV Zovirax in high doses for herpetic encephalitis, it is necessary to monitor renal function, especially if it is initially impaired or there is dehydration.
Patients taking high doses of Zovirax by mouth should receive sufficient fluids.
The prepared Zovirax solution has a pH of 11.0 and cannot be used orally.
Patients should be informed of the possibility of a transient mild burning sensation after application of Zovirax ophthalmic ointment. During treatment with the drug, patients should not wear contact lenses.
Zovirax analogs
Level 4 ATX code matches:
Aldara
Viru-Merz Serol
Zovirax Duo-Active
Acyclovir
Fenistil Pencivir
Bonafton
Atsik
Alpizarin
Herpferon
Epigen Intim
Lysozyme
Gerpevir
Kondilin
Virolex
Gevisosh
Cheaper analogues of Zovirax are listed below: Acigerpin (lip analogue), Acyclovir (cream, eye ointment, tablets), Herperax (ointment), Acyclovir Belupo (tablets, cream), Acyclovir Sandoz (tablets, cream), Acyclovir-Acri (tablets , ointment), Acyclovir-Akrikhin (tablets, ointment), Acyclostad (cream), Vivorax (cream), Virolex (cream, lyophilisate, eye ointment, tablets), Medovir (lyophilisate), Acivir (cream), Herpetad (cream), Provirsan (tablets), Zovirax Duo (cream).
For children
Zovirax tablets
Treatment and prevention of infectious lesions caused by the common herpes virus in pediatric patients with immunodeficiency :
- up to the age of 2 years, use half the dosage for adults;
- over 2 years of age, use adult doses described in the section “Instructions for use of Zovirax” .
For the treatment of chickenpox in children, the following dosages are used:
- children under 2 years of age are prescribed 200 mg four times a day;
- children 2-6 years old are prescribed 400 mg four times a day;
- Children over 6 years old are prescribed 800 mg four times a day.
More accurately, the dosage can be determined based on the child’s weight: 20 mg/kg body weight four times a day. The course of therapy is 5 days.
According to the limited information currently available, adult dosages of Zovirax are permitted immunodeficiency “Instructions for use of Zovirax” ).
Zovirax eye ointment and cream
Zovirax eye ointment and cream are used by aunts according to the same scheme and in the same doses as adults (described in the section “Instructions for use of Zovirax”).
Lyophilisate for the preparation of injections
Dosages for intravenous administration in children 3 (months) - 12 (years) are calculated based on body area.
When treating infectious lesions associated with the herpes simplex virus (excluding herpetic encephalitis ) and herpes zoster virus , doses of intravenous infusions are calculated according to the scheme 250 mg/m2 three times a day.
In the treatment of infectious lesions associated with the herpes zoster virus and herpetic encephalitis in sick children with immunodeficiency doses are calculated according to the regimen of 500 mg/m2 three times a day.
It is suggested that children 2 years of age and older who have received a bone marrow transplant can be given adult doses of Zovirax.
Clinical pharmacology
In patients with severe immunodeficiency, long-term or repeated courses of acyclovir therapy may lead to the emergence of resistant strains, and therefore further treatment with acyclovir may be ineffective. The majority of isolated strains with reduced sensitivity to acyclovir had a relatively low content of viral thymidine kinase and a disorder in the structure of viral thymidine kinase or DNA polymerase. The effect of acyclovir on HSV strains in vitro can also lead to the formation of strains that are less sensitive to it. A correlation has not been established between the sensitivity of HSV strains to acyclovir in vitro and the clinical effectiveness of the drug.
It has been shown that intravenous administration of Zovirax in high doses reduces the incidence and delays the development of CMV infection. If, after infusion therapy with Zovirax in a high dose, treatment with Zovirax for oral administration in a high dose is carried out for 6 months, then mortality and the incidence of viremia are reduced.
Reviews of Zovirax
Almost all reviews of Zovirax ointment and reviews of other forms of release of this drug perfectly characterize the therapeutic effectiveness of the drug. Zovirax is most often used for herpes . Reports of side effects and ineffectiveness of the drug are extremely rare. The greatest dissatisfaction of patients is the high price of the drug.
Which is better: Zovirax or Acyclovir?
Acyclovir and Zovirax have the same active substance and release forms. Based on reviews, when used for herpes , there is no difference in effect, given that the latter costs almost 20 times more. The choice between drugs should be made based on economic considerations and individual characteristics.
Zovirax price, where to buy
Zovirax price in tablets
A package of the drug 200 mg No. 25 in Russia costs from 500 to 850 rubles. In Ukraine, its average price is 90 hryvnia.
How much does the ointment cost?
The price of Zovirax ointment in Russia is 270-380 rubles. There are no data on the price of ointment in Ukraine.
Cream price
The price of Zovirax cream (5 g), used for colds on the lips, in Russia ranges from 180-195 rubles. In Ukraine, a tube of cream (2 g) will cost approximately 55-75 hryvnia.
Zovirax lyophilisate price
The price of a package of lyophilisate 250 mg No. 5 in Russian pharmacies costs 1590-1750 rubles. In Ukraine, such packaging can be purchased for 670-740 hryvnia.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Zovirax tablets 200 mg 25 pcs. Glaxo Wellcome S.A./GlaxoSmithKline Pharmaceuticals S.A.
248 rub. order - Zovirax Zovirax for colds on the lips, cream 5%, 5gGlaxo Welcome Operations
RUB 193 order
- Zovirax Lyof. for prig solution for inf. 250 mg 5 pcs GlaxoSmithKline SpA
RUB 1,325 order
Pharmacy Dialogue
- Zovirax for cold lips, cream 5% 5gGlaxo-Wellcome
182 RUR order
- Zovirax DUO-ACTIVE for colds on the lips, cream 5% 2gGlaxo-Wellcome
428 RUR order
- Zovirax tablets 200 mg No. 25GlaxoSmithKline Pharmaceuticals CA
RUB 253 order
- Zovirax cream (tube 5% 5g)Glaxo-Wellcome
RUB 198 order
- Zovirax (200 mg tablet No. 25) Glaxo Wellcome S.A.
RUB 246 order
show more
Pharmacy24
- Zovirax 200 mg No. 25 tablets Glaxo Wellcome S.A.
Spain/GlaxoSmith Klein Pharmaceuticals S.A., Poland 96 UAH order - Zovirax 250 mg No. 5 lyophilisate for the preparation of solution for infusion GlaxoSmithKline Manufacturing S.p.A., Italy
801 UAH order
- Zovirax 5% 2 g cream Glaxo Operations UK Limited, Great Britain
50 UAH order
- Zovirax Duo 2 g cream Glaxo Operations UK Limited, Great Britain
68 UAH order
PaniPharmacy
- Zovirax infusion Zovirax lyophilisate for solution for infusion 250 mg No. 5 Italy, GlaxoSmithKline Manufacturing
924 UAH. order
- Zovirax cream Zovirax cream 5% 2g UK, Glaxo Operations UK
52 UAH order
- Zovirax tablets Zovirax tablets 200 mg No. 25 Poland, GlaxoSmithKline Pharmaceuticals
101 UAH order
- Zovirax ointment Zovirax ointment for eyes. 3% 4.5g Canada, Jubilant HollisterStier General Partnership
248 UAH order
show more