06/07/2018 I’ll start today’s article with what has already become a classic situation.
A person comes to see a doctor (dermatologist or oncologist) and shows him his moles.
And then something like this happens:
– You have dysplastic nevi here, here and here.
- What is this? Is it dangerous?!
– Dysplastic nevi are moles with an uneven edge and uneven color. Dysplastic nevi can develop into melanoma and must be removed.
- Oh!!! How scary!!! Let's delete it quickly!!!
The moles are safely removed and sent for histology.
A person comes home and starts reading on the Internet about dysplastic nevi. And then they add fuel to the fire:
- A dysplastic nevus is any nevus with an uneven edge and uneven coloration.
- All dysplastic nevi turn into melanoma in 146% of cases.
- To prevent death from melanoma, you need!!! URGENTLY!!! remove all dysplastic nevi.
All this “beauty” has been copied from one site to another for many years and fills the domestic segment of the Internet to the brim.
Let's understand all this from the point of view of evidence-based medicine.
How dangerous are dysplastic nevi?
There are specific, proven figures here that it would be better for those who spread hysteria on the “Internet” to become familiar with.
- The annual risk of a dysplastic nevus turning into melanoma is 1 in 10,000. According to the authors [4], this is very low.
- About 70% of melanomas develop not against the background of nevi, but against the background of unchanged skin [5].
- A study examining the genetic profile of atypical nevi has challenged the hypothesis that they are precursors to melanoma [6].
- In two studies, the authors followed patients with incompletely removed, histologically confirmed dysplastic nevi for a long time (up to 17 years) [8,9]. Based on this, the very thesis about the increased risk of developing melanoma from a dysplastic nevus can be seriously questioned.
Causes
How common is giant congenital melanocytic nevus? It occurs in approximately 1 in 20,000 newborns worldwide.
What genetic changes are responsible for its occurrence? In most cases, these are mutations of the NRAS gene, less often - mutations of the BRAF gene.
The proteins produced under the influence of these genes are involved in the process of signal transmission from outside the cell to the cell nucleus. These signals command cells to grow and divide (proliferate) or to mature and perform a specific function (differentiation). For signal transmission, the NRAS and BRAF proteins must be activated, otherwise the signal will not pass.
Mutations of the NRAS and BRAF genes, which are responsible for the formation of giant congenital melanocytic nevus, are somatic. This means that they can be acquired throughout life and only exist in certain cells. These mutations appear during embryonic development, during the growth and proliferation of cells - future melanocytes.
Somatic mutations in NRAS and BRAF cause the protein in the affected cells to be in the “on” state all the time, which means it constantly transmits signals. Excessive protein activity leads to uncontrolled growth and division of melanocytes and, as a consequence, the development of a giant congenital melanocytic nevus. This process occurs during intrauterine development.
What to do? Should I delete or not?
Fortunately, I was not able to find practical recommendations in which the mere presence of an atypical nevus on a person’s skin would be considered a direct indication for its removal.
On the other hand, the presence of single or multiple, atypical [7] and dysplastic [3] nevi on a person’s skin equally increases the risk of developing melanoma on the skin as a whole.
Thus, in my opinion, the most logical tactic seems to be not prophylactic removal of atypical nevi, but increasing the frequency of preventive self-examinations and examinations by an oncologist.
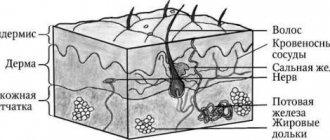
The structure of the skin
The skin is the largest integral multifunctional organ, interconnected with all other organs and systems of the body. In direct contact with the external environment, it performs a barrier-protective function. On the surface of the skin there is a complex pattern in the form of triangular and rhombic fields, formed by numerous grooves. Coarser grooves form folds in the palms, soles, scrotum, and facial wrinkles.
Histologically, three layers of skin are distinguished (Fig. 1):
- epidermis;
- derm (dermis);
- subcutaneous fatty tissue (subcutis), or hypodermis (hypodermis).
Rice. 1. Skin structure
The epidermis is the epithelial part of the skin, and the dermis and hypodermis are connective tissue. The border zone between the epidermis and dermis has the appearance of a wavy line due to the presence of outgrowths in the dermis - papillae, which cause the formation of ridges and furrows on the surface of the skin, forming a skin pattern. The connective tissue part of the skin (dermis and hypodermis) contains nerves, blood and lymphatic vessels, and muscles. In addition, the skin has its own appendage structures, which include hair, sebaceous and sweat glands, as well as nails.
- Gallery
- News
- Reviews
- Vacancies
- Licenses
- Insurance partners
- Controlling organizations
- Schedule for receiving citizens for personal requests
- What you need to know about coronavirus infection?
- Rules for patients
- Important! Visiting a clinic during self-isolation
- Online consultation with a doctor
- to corporative clients
- Documentation
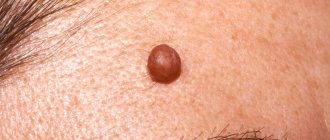
Dysplastic nevus is one of the types of flat pigmented skin formations, which are more often called moles.
Patients, and many doctors, mistakenly believe that all flat pigmented formations are benign and do not pay attention to them. However, it is known that dysplastic nevi can transform into melanoma. The transformation process occurs gradually as lentiginous melanocytic dysplasia (LMD) develops from grade 1 to grade 3.
This type of formation deserves the closest attention among all types, since there is a very high risk of their malignancy with transition to the most malignant tumor - melanoma. The term dysplastic itself means that it is a nevus that is not similar in external and internal characteristics to typical moles. Dysplastic nevus, which occurs in 5%-9% of the white population, has recently attracted the attention of researchers, as it may be a precursor to superficial spreading melanoma: it is found in almost all patients with hereditary melanoma and in 30–50% of patients with sporadic melanoma . A dysplastic nevus can occur on intact skin or be a component of a complex noncellular nevus.
Clinically, dysplastic nevus is similar to borderline nevus, but there are also differences. Thus, a dysplastic nevus is an irregularly shaped spot, while a borderline nevus has a regular shape - round or oval. The color of a dysplastic nevus is often heterogeneous, with areas of dark pigment, while a borderline nevus is characterized by a uniform color, the color of both nevi varies from light brown to dark brown. Often, a pinpoint formation resembling a target is noted in the center of a dysplastic nevus. When a dysplastic and mixed nevus is combined, there is a picture of a “fried egg” with a raised yolk in the center.
In our observations, dysplastic nevi were found in 5% - 10% of patients who consulted a surgeon or oncologist for various diseases. In some, the nevi were single - from 3 to 10 formations, in others - multiple - from 50 to 100 or more. Observing patients with multiple dysplastic nevi, we identified 2 types of these formations.
In the first type - there were fewer of these patients - dysplastic nevi appeared in childhood and adolescence, were often hereditary, but these patients did not report melanoma in relatives. Carriers of nevi were, as a rule, white-skinned, poorly tanned, with blond or red hair and light eyes. Dysplastic nevi of this type were large - 0.5 - 1.0 cm in diameter, located on open and closed areas of the body (buttocks, lower abdomen), and were often combined with papillomatous nevi. The color of nevi in the same patient could vary from pink to dark brown, sometimes a variegated color was observed: dark areas on a pink or light brown background. This pattern of nevi has been described as “dysplastic nevus syndrome.” After puberty, new nevi did not appear in this group of patients. This fact can be attributed to the fact that white-skinned people limited their exposure to the sun, as they quickly burned, and were also informed about the possible appearance of new “moles” as a result of exposure to ultraviolet radiation.
In the second type, dysplastic nevi were rare in adolescence; most nevi appeared in adulthood and were associated with frequent and prolonged exposure to the sun while vacationing in southern latitudes. Dysplastic nevi of this type were small - from 0.1 to 0.4 cm in diameter, regular rounded in shape, uniform in color, combined with multiple freckles in young patients and pigment spots of the “Dubreuil’s melanosis” type in older patients. In white-skinned patients with skin phototype 1 - 2, the nevi were light brown, in others they were brown or dark brown. The density of nevi was higher on sun-exposed areas of the body: the face, forearms, outer surface of the shoulders, upper half of the back and chest wall (like a “wide neckline”). All this indicates some similarity between dysplastic nevi of the second type and Dubreuil’s melanosis, which is a proliferation of melanocytes in the basal layer of the epidermis in individuals with skin phototype 1 - 2 under the influence of repeated sunburn.
According to our observations, the most important sign of progression of dysplastic nevus is:
- The appearance of pigment formation on unchanged skin and its further growth over several months or years in persons over 18 years of age , i.e. in adulthood.
- Changes in the last 3-5 years of a long-existing nevus may indicate the progression of a dysplastic nevus, which is combined with a borderline or mixed nevus.
- An important sign of a progressive dysplastic nevus is a very dark color (almost black), or uneven coloration of the formation with areas of dark brown or black.
- The irregular shape of the nevus may be little noticeable with a small size of the progressive dysplastic nevus , at the same time it can also be observed in a long-existing noncellular nevus - borderline or mixed.
Excision of dysplastic nevus
Excision of the nevus (excisional biopsy) should be performed under local anesthesia, 0.4–1.0 cm from the visible boundaries, with subcutaneous tissue. Histological examination of a removed nevus should be carried out by a pathologist with experience in the study of melanocytic formations. Progressive dysplastic nevi require excision for prevention purposes, as well as early diagnosis of cutaneous melanoma . When melanoma is detected, the question of the need for reoperation - excision of the postoperative scar - is decided depending on the thickness of the tumor, determined by histological examination. According to foreign authors, as well as WHO recommendations, an adequate deviation from the boundaries of the formation for in-situ melanoma is 0.2 - 0.5 cm, for invasive melanoma with a thickness of less than 1.5 mm - 1.0 cm.
During removals carried out in the department of surgery from June 2009 to April 2014, of all removed pigmented formations, the clinical diagnosis of “dysplastic nevus” was confirmed morphologically in 76% of cases, i.e. Histological examination of these formations revealed structures of lentiginous melanocytic dysplasia (LMD). In 2.4% of cases, melanoma was detected that developed against the background of LMD ; in other cases, other types of nevi were confirmed (intradermal, mixed nevus, nevus of the sebaceous glands). In our clinical cases, patients with diagnosed melanoma did not require reoperation.
Foreign authors recommend removing dysplastic nevi 0.6 cm or more; according to our research, smaller nevi - 0.4 - 0.5 cm in diameter - can also be removed. In our practice, melanoma in one case measured 0.5 x 0.4 cm, in another - 0.5 cm.
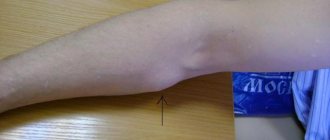
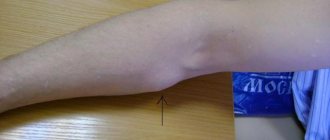
Photos 1 and 2 show dysplastic nevi with signs of progression.
Photo 1 shows a nevus of the gluteal region measuring 0.5 x 0.4 cm in a 29-year-old patient with skin phototype 1. The nevus, according to the patient, appeared 7 months ago in the form of a punctate formation, which gradually increased (a sign of progression) . No other pigmented nevi or freckles were found on the body. Histological examination: lentiginous pigmented nevus with severe (grade 3) melanocytic lentiginous dysplasia.
Photo 2 shows a nevus of the chest wall in a 27-year-old patient , 0.3 cm in diameter, round in shape, which darkened after staying in the south (a sign of progression) . Histological examination - mixed dysplastic pigmented nevus with severe (grade 3) melanocytic lentiginous dysplasia.
If the patient refuses surgery or in the absence of obvious signs of progression of the dysplastic nevus, the nevus should be re-examined by a doctor after 6 months. If there are changes in the nevus over the past period, surgery is recommended; if there are no changes, further observation after 6 months.
Patients with dysplastic nevi need:
- avoid prolonged exposure to direct sunlight,
- protect the skin with clothing,
- apply sunscreen.
The material was prepared based on the article: “Tactics for managing a patient with a dysplastic nevus” - O.A. Romanova, N.G. Artemyeva, E.A. Yagubova, I.M. Rudakova, V.N. Marycheva, A.A. Veshchevaylov . Clinical dermatology and venereology No. 2, 2015.
Services and prices
Histological examination of skin biopsy material
1,650 rub.
Appointment with a dermatovenerologist, therapeutic and diagnostic, primary, outpatient
2,000 rub.
Appointment with a dermatovenerologist, therapeutic and diagnostic, repeat, outpatient
1,800 rub.
Appointment with an oncologist, treatment and diagnostic, primary, outpatient
2,000 rub.
Appointment with an oncologist, therapeutic and diagnostic, repeated, outpatient
1,800 rub.
Appointment with a surgeon LD, PERV, AMB
2,000 rub.
Appointment with a surgeon, candidate of medical sciences, primary, outpatient
2,500 rub.
Removal of benign formations with a diameter of up to 5 mm using the molecular resonance surgical device VESALIUS LX 80 (with anesthesia and bandage)
2,500 rub.
Removal of a benign tumor with a diameter of more than 5 mm using a molecular resonance surgical device VESALIUS LX 80 (with anesthesia and bandage)
3,100 rub.
Isaeva Galina Ivanovna Oncologist Work experience: 18 years
Romanova Olga Aleksandrovna Oncologist (mammologist) Work experience: 62 years
Artemyeva Nadezhda Georgievna Head of department, surgeon, phlebologist Doctor of the highest category Work experience: 43 years
Bezlepko Marina Gennadievna Oncologist Work experience: 12 years