Diagnostics
If the patient does not have the main symptoms of purpura (bleeding and hemorrhage), doctors have difficulty establishing the correct diagnosis.
You need to be especially careful not to confuse purpura with vascular skin abnormalities. Purpura is diagnosed, as a rule, according to the clinical and hematological picture. First, a hematologist examines the patient. To confirm the diagnosis, the doctor prescribes the patient to undergo the following hardware examinations and tests:
- general clinical and biochemical blood tests;
- urea test;
- general clinical urine test;
- myelogram.
Also, to establish purpura, differential diagnosis is carried out by identifying symptoms of diseases such as hemolytic-uremic syndrome, hemolytic microangiopathic purpura and hepatorenal syndrome.
As a rule, purpura is treatable, but there are cases of death if the bleeding and hemorrhage are very heavy.
Henoch-Schönlein purpura
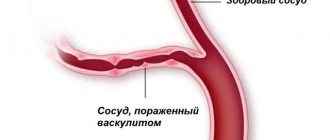
Henoch-Schönlein purpura (hemorrhagic vasculitis) is a systemic vasculitis affecting the microvasculature (arterioles, capillaries and post-capillary venules), with typical deposition in the vascular wall of immune complexes consisting of immunoglobulin A (IgA). Clinically, the disease manifests itself as a hemorrhagic skin rash, joint syndrome, damage to the gastrointestinal tract (GIT) and kidneys.
Henoch-Schönlein purpura develops at any age, but the maximum incidence is observed in children aged 4-6 years, amounting to approximately 13-18 cases per 100 thousand. With age, the incidence decreases and the development of the disease after 60 years is considered rare.
The etiology of the disease has not been established, however, an increase in the incidence of Henoch-Schönlein purpura in the cold season, as well as the frequent association of the onset of the disease with episodes of acute respiratory or intestinal infection may indirectly indicate the infectious nature of the disease. The list of etiological agents associated with the development of Henoch-Schönlein purpura includes β-hemolytic streptococcus group A, Haemophilus influenzae, chlamydia, mycoplasma, Legionella, Yersinia, Epstein-Barr, Coxsackie, hepatitis B and C viruses, adenovirus, cytomegalovirus, parvovirus B19 , salmonella, Helicobacter pylori, Clostridium difficile. There are isolated observations of Henoch-Schönlein purpura that developed after vaccination against typhoid fever, measles, and influenza. Alcohol, medications, food products, hypothermia, and insect bites can be triggers for the disease.
Pathogenesis
Currently, Henoch-Schönlein purpura is considered an immune complex disease associated with the deposition of granular IgA deposits in the vascular wall and activation of complement. This concept is based on the results of numerous studies showing impaired synthesis and/or metabolism of IgA in the majority of patients with Henoch-Schönlein purpura: increased levels of serum IgA, IgA-containing immune complexes, and IgA-fibronectin complexes. However, the pathogenetic significance of these disorders requires further evaluation. In recent years, evidence has accumulated indicating the anti-inflammatory properties of IgA, which gives grounds to regard the increase in its synthesis as a compensatory process that occurs secondarily in response to an inflammatory reaction in the mucous membranes. Thus, it has been shown that IgA has the ability to reduce the production of proinflammatory cytokines and is not able to activate complement; IgA is found in the endothelium of unaffected vessels and in the mesangium of unchanged renal glomeruli; The observation of Henoch-Schönlein purpura with complete selective IgA deficiency is described. Considering the frequent association of the development of Henoch-Schönlein purpura with episodes of infections of the respiratory tract and gastrointestinal tract, this assumption seems quite probable.
Another reason for changes in IgA metabolism in Henoch-Schönlein purpura may be a violation of O-glycosylation of the hinge region of the heavy chains of the IgA1 molecule, which, as has been shown, can lead to impaired clearance of IgA1 by liver receptors and prolongation of the circulation period of IgA polymers and IgA-containing immune cells. complexes in the systemic circulation. It has been shown that IgA1 molecules with aberrant glycosylation acquire the ability to activate complement via an alternative pathway and have an increased tropism for the mesangial matrix of the renal glomeruli.
In recent years, additional data have been obtained that indirectly confirm the assumption about the infection-dependent nature of Henoch-Schönlein purpura. Thus, it was shown that in the majority of patients during an exacerbation of cutaneous vasculitis, transient endotoxemia is observed - circulation of lipopolysaccharide of gram-negative bacteria in the systemic bloodstream. The pathogenetic significance of this phenomenon in Henoch-Schönlein purpura requires further study, but the possibility of endotoxin involvement in the development of vascular inflammation mediated by the Schwartzman reaction is assumed. Chronic inflammation of the intestinal wall, possibly caused by dysfunction of its local immune system or an infectious process, can play an important role in the pathogenesis of endotoxemia. This assumption is supported by the discovery of increased intestinal permeability for macromolecules (ovalbumin) in the majority of patients with Henoch-Schönlein purpura during exacerbations of cutaneous vasculitis. In addition, the presence of a chronic inflammatory process in the mucous membrane of the small intestine in patients with Henoch-Schönlein purpura has been demonstrated, which, apparently, is the morphological basis for dysfunction of the intestinal barrier and the development of transient endotoxemia.
Clinical picture
The clinical picture of Henoch-Schönlein purpura consists of four typical manifestations: hemorrhagic skin rash, damage to the joints, gastrointestinal tract and kidneys. In most cases, the disease develops gradually, gradually and does not significantly affect the general condition of the patients. As a rule, this variant of the onset of the disease is observed with isolated skin lesions. The number of organ manifestations of Henoch-Schönlein purpura varies from 1-2 to a combination of all 4 classical signs, which can develop in any order over several days or weeks of illness. In some cases, in addition to the mentioned manifestations, damage to other organs, in particular the lungs, heart, and central nervous system, may develop.
Skin lesions are observed in all patients with Henoch-Schönlein purpura and are a mandatory diagnostic criterion. In most cases, a hemorrhagic rash is the first clinical manifestation of the disease, which is subsequently accompanied by damage to other organs and systems. The most typical localization of skin rashes: lower extremities - legs and feet. Often the skin rash spreads to the thighs, buttocks, torso, upper limbs and extremely rarely to the face. In the process of evolution, hemorrhages gradually become pale, transform into brown pigment spots and then disappear. With a long-term recurrent course, the skin in the affected area may become pigmented due to the development of hemosiderosis. In most cases, the hemorrhagic rash is represented by petechiae and purpura, but in some cases erythematomacular and urticarial elements may also be observed.
Joint damage, as a rule, develops in parallel with skin damage and occurs as migratory polyarthralgia, less often - arthritis. The favorite localization is the knee and ankle joints; the elbow, wrist and other joints are less commonly affected. These manifestations of the disease are always transient and benign, never leading to the development of permanent changes in the joints.
Damage to the gastrointestinal tract is observed in 60-80% of childhood patients and 40-65% of adult patients. The most constant symptom: abdominal pain, worsening after eating, which often creates a typical picture of “abdominal toad.” A common complication of abdominal lesions in Henoch-Schönlein purpura is intestinal bleeding.
Kidney damage in Henoch-Schönlein purpura can become chronic and is the main factor determining the prognosis of the disease as a whole. The incidence of renal involvement varies from 30 to 70% depending on the age of the patients. In adults, kidney damage is detected almost 2 times more often than in children. As a rule, clinical signs of kidney damage are detected in the first 3 months of the disease, however, with a chronic recurrent course of cutaneous vasculitis, delayed appearance of signs of glomerulonephritis is possible - several months or even years after the onset of the disease. Possible predictors of renal involvement in children include male gender, age over 5 years, abdominal syndrome, persistent cutaneous purpura, and decreased plasma levels of factor XIII. In adult patients, risk factors for kidney damage include the presence of fever and episodes of infections at the onset of the disease, the spread of skin rash on the torso, severe abdominal syndrome and the presence of laboratory signs of inflammatory activity of the disease. The severity of renal pathology, as a rule, does not correlate with the severity of skin manifestations of the disease, however, in both children and adults, a significant positive correlation was noted between the frequency of kidney damage and the development of abdominal syndrome, which requires more careful dynamic monitoring of the corresponding cohort of patients. In children, in half of the cases, kidney damage has a favorable course with complete clinical and laboratory recovery, while in most adult patients there is a tendency towards a chronic persistent course of nephritis.
In half of patients with Henoch-Schönlein purpura, glomerulonephritis is manifested by microhematuria, which, as a rule, is combined with minimal or moderate proteinuria. A third of patients experience gross hematuria, which most often develops at the onset of nephritis, but can also occur at later stages of renal damage during exacerbations of cutaneous vasculitis or respiratory infections. More severe types of renal damage are also possible, including nephrotic syndrome, rapidly progressive nephritis and acute renal failure. Arterial hypertension syndrome is detected in 14-20% of patients. The development of chronic renal failure (CRF) as a result of glomerulonephritis is observed in 12-30% of patients.
Diagnostics
Diagnosis of Henoch-Schönlein purpura is based on identifying typical clinical signs of the disease, primarily bilateral hemorrhagic skin rashes at the time of examination or in the medical history. There are no specific laboratory tests for Henoch-Schönlein purpura. Changes in the clinical blood test - an increase in the erythrocyte sedimentation rate (ESR) - may reflect the inflammatory activity of the disease, as well as the severity of complications (anemia due to intestinal bleeding). The presence of thrombocytopenia is a criterion for excluding Henoch-Schönlein purpura. A marked increase in ESR and significant dysproteinemia are not typical for Henoch-Schönlein purpura. Disease activity is reflected by the level of von Willebrand factor and thrombomodulin in the blood plasma. The detection of high levels of fibrin/fibrinogen degradation products in plasma in active forms of the disease is not a sign of the development of DIC syndrome, but only reflects the high inflammatory activity of the disease. The examination plan for all patients must include a virological and immunological blood test to exclude other diseases that occur with skin purpura.
A key role in confirming the clinical diagnosis is played by a biopsy of the skin and/or kidneys, less often of other organs, with the obligatory immunohistochemical study revealing the fixation of IgA-containing immune complexes in the vascular wall. It should be taken into account that in addition to Henoch-Schönlein purpura, IgA deposits are found in skin lesions as part of chronic inflammatory bowel diseases (Crohn's disease, ulcerative colitis), chronic diffuse liver diseases of alcoholic etiology, celiac disease, and Dühring's dermatitis herpetiformis.
The morphological picture of kidney damage in Henoch-Schönlein purpura is identical to that in Berger's disease (primary IgA nephropathy). The most common morphological variant of kidney damage is mesangioproliferative glomerulonephritis, characterized by focal or diffuse proliferation of mesangiocytes. Immunohistochemical examination reveals granular deposits of IgA, less often IgG, as well as the C3 component of complement, fibrin. In more severe cases, the formation of epithelial “crescents” is noted.
Widely used classification diagnostic criteria for Henoch-Schönlein purpura, proposed in 1990? American College of Rheumatology and including the patient's age less than 20 years, palpable purpura, abdominal syndrome and the morphological picture of cutaneous leukocytoclastic vasculitis (2 or more of 4 criteria are required), have little practical significance due to their low sensitivity and specificity (87.1 and 87. 7%, respectively).
Differential diagnosis
Differential diagnosis is carried out with a wide range of diseases that occur with damage to small vessels:
- primary vasculitis of small vessels (Wegener's granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome, cryoglobulinemic vasculitis). The results of blood tests for antineutrophil cytoplasmic antibodies (Wegener's granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome) and cryoglobulins (cryoglobulinemic vasculitis) have differential diagnostic significance; morphological examination data (granulomatous inflammation in Wegener's granulomatosis, eosinophilic vasculitis in Churg-Strauss syndrome). Of particular importance is the immunohistochemical examination of biopsy specimens of affected tissues. Detection of IgA deposits is a characteristic sign of Henoch-Schönlein purpura.
- Vasculitis in autoimmune diseases (systemic lupus erythematosus, rheumatoid arthritis, Sjogren's disease, Crohn's disease, ulcerative colitis). Differentiation is based on the clinical features inherent in each nosology, the results of laboratory and instrumental research methods.
- Vasculitis in infections (subacute infective endocarditis, tuberculosis, infection with hepatitis B and C viruses), malignant neoplasms, drug allergies.
Treatment
For skin lesions, the following drugs from the sulfonamide group can be effective: sulfasalazine (orally, 500-1000 mg 2 times a day), colchicine (orally, 1-2 mg once a day). Glucocorticoids are effective in the vast majority of patients, especially in high doses, but their long-term use in patients with Henoch-Schönlein purpura without involvement of internal organs is undesirable, since the severity of side effects in such a situation may exceed the severity of the disease itself. The prescription of non-steroidal anti-inflammatory drugs (NSAIDs) is justified only in cases of severe articular syndrome and the ineffectiveness of other drugs. In other cases, prescribing NSAIDs should be avoided due to the adverse effect on the intestinal mucosa and increased intestinal permeability.
Damage to the gastrointestinal tract with intense abdominalgia is an absolute indication for the use of glucocorticoids:
prednisolone intravenous drip 300-500 mg/day for 3 days in a row, followed by switching to oral administration 0.5 mg/kg 1 time per day for 2-3 weeks, then quickly reducing the dose by 5? mg every 3 days to full cancellations.
Gastrointestinal bleeding (if it is caused by vasculitis and not other reasons) is not a contraindication to the administration of oral glucocorticoids, but, on the contrary, serves as one of the main indications for such treatment. A contraindication to the administration of glucocorticoids orally for abdominal syndrome can only be perforation of the intestinal wall, which currently extremely rarely complicates the course of Henoch-Schönlein purpura.
The greatest problems in the drug treatment of Henoch-Schönlein purpura are associated with the choice of treatment for chronic glomerulonephritis. Most authors consider the use of ultra-high doses of glucocorticoids, cytostatics and/or plasmapheresis sessions to be justified in cases of severe glomerulonephritis (nephrotic syndrome with normal or impaired renal function; more than 50% of glomeruli with epithelial “crescents”).
In this case, the following scheme is used:
- prednisolone orally 1 mg/kg 1 time per day for 4-6 weeks, then reduce the dose by 2.5 mg/week until complete withdrawal or prednisolone intravenous drip 15 mg/kg 1 time per day for 3 consecutive days (total 6-20 three-day “pulses” with an interval of 3-4 weeks); +
- cyclophosphamide intravenous drip 15 mg/kg once every 3-4 weeks, under the control of the level of peripheral blood leukocytes and transaminases (6-20 “pulses” in total); +
- plasmapheresis with an exfusion volume of 30-60? ml/kg, 10-14 sessions.
Single uncontrolled studies have shown the effectiveness of a combination of glucocorticoids and azathioprine in severe cases of kidney damage, as well as a combination of glucocorticoids and cyclophosphamide with antiplatelet agents or anticoagulants.
In addition, for the treatment of patients with nephrotic and rapidly progressive glomerulonephritis, it is proposed to use intravenous immunoglobulins:
normal human immunoglobulin intravenously at 400-1000 mg/kg for 1-5 days, repeated courses once a month for 6 months.
There is no consensus regarding less severe forms of glomerulonephritis. With isolated microhematuria, minimal proteinuria (up to 0.5 g/day) and preserved renal function, as a rule, active immunosuppressive treatment is not required. For moderate proteinuria (0.5-1 g/day), the prescription of drugs that affect the non-immune mechanisms of progression of kidney damage is indicated: angiotensin-converting enzyme inhibitors and/or angiotensin II receptor antagonists (due to their ability to reduce intraglomerular hypertension and the severity of proteinuria), statins (for lipid metabolism disorders). Some retrospective studies have shown a beneficial effect of tonsillectomy on the course of mild forms of glomerulonephritis in Henoch-Schönlein purpura.
Correction of hemostasis disorders, previously considered a priority in the treatment of Henoch-Schönlein purpura, is currently considered only as an auxiliary method of therapy, the prospects of which are assessed with skepticism. In practical terms, the reports of Japanese researchers on the favorable long-term clinical and pathomorphological effect of fibrinolytic therapy with urokinase on the course of glomerulonephritis in Henoch-Schönlein purpura are of interest:
urokinase intravenously slowly 5000 IU/kg 3 times a week for 3-12 weeks.
According to the authors, the action of urokinase may be based on reducing the severity of intraglomerular hypercoagulation and dissolving fibrinogen/fibrin deposits.
Forecast
An important clinical prognostic factor that determines the incidence of chronic renal failure is the severity of proteinuria. So, if with minimal proteinuria chronic renal failure develops in 5% of patients, then with nephrotic syndrome this figure increases to 40-50%. The most unfavorable with regard to the development of chronic renal failure is the combination of nephrotic syndrome with arterial hypertension and impaired renal function at the onset of glomerulonephritis.
The most important morphological criterion for determining the prognosis of kidney damage is the proportion of renal glomeruli with “crescents” from the total number of glomeruli. Thus, according to French authors who observed 151 patients from 1 year to 18 years, in the presence of “crescents” in more than 50% of the glomeruli, end-stage renal failure developed in 37% of patients, and in another 18% glomerulonephritis had a chronic progressive course. On the other hand, 85% of patients who achieved end-stage renal disease had crescents in more than half of the glomeruli. In 70% of patients with complete recovery or minimal changes in urine tests, no “crescents” were found in the glomeruli.
It is important that the majority of patients with late progression of glomerulonephritis do not have clinical signs of activity of renal and extrarenal lesions, which is explained by the predominant influence of non-immune mechanisms of progression on the course of renal damage. In this regard, in all patients with Henoch-Schönlein purpura with kidney damage, careful monitoring of blood pressure and correction of metabolic disorders, in particular hyperuricemia and dyslipidemia, are extremely important.
Treatment
The essence of the treatment of purpura is to reduce the amount of production of antiplatelet antibodies and make it impossible for them to bind to platelets.
If the patient experiences bleeding, the doctor prescribes a course of hemostatic drugs, as well as aminocaproic acid. If the bleeding is also uterine, then the patients are prescribed oxytocin. In the event that there is a risk of hemorrhage in the brain, surgeons prescribe platelet infusion (infusion) to the patient.
There is also a surgical method for treating purpura. In this case, the patient undergoes surgery to remove the spleen (splenectomy). But it is very important to remember that surgical interventions are an extreme case when conservative therapy is ineffective.
Another method of treating purpura is plasmapheresis, or a procedure for purifying blood plasma from antibodies and toxins by filtering the blood plasma using special devices.
Doctors also advise patients with vascular purpura to follow a hypoallergenic diet.
Henoch-Schönlein disease
Skin syndrome is the most common. It symmetrically affects the limbs, buttocks, and less commonly the torso. A papular-hemorrhagic rash occurs, sometimes with blisters. The rashes are of the same type, at first they have a distinct inflammatory basis, in severe cases they are complicated by central necrosis and become covered with crusts, leaving pigmentation for a long time. When pressed, the elements of the rash do not disappear.
Joint syndrome often occurs together with skin syndrome or several hours or days after it in the form of pain of varying intensity in large joints (knees, elbows, hips). After a few days, the pain goes away, but with a new wave of rashes it can occur again. In some cases, articular damage is persistent and stubborn, reminiscent of rheumatoid polyarthritis.
Abdominal syndrome is more often observed in childhood (in 54-72% of patients), in approximately 1/3 it predominates in the clinical picture, in some cases it precedes skin changes, which makes diagnosis very difficult. The main symptom is severe abdominal pain, constant or cramping, sometimes so intense that patients cannot find a place in bed and scream for many hours. The pain is caused by hemorrhages in the intestinal wall. These hemorrhages can be combined with blood soaking of the intestinal wall and mucous membrane, bleeding from it and from areas of necrosis, bloody vomiting, melena (admixture of blood in the stool) or fresh blood in the stool, as well as false urges with frequent stools or, conversely, with its delay. From the very beginning, fever and more or less pronounced leukocytosis (an increase in the number of leukocytes in the blood) are detected. With heavy bleeding, collapse (fainting) and acute posthemorrhagic anemia develop. In some cases, frequent vomiting leads to a large loss of fluid and chloride. The coagulogram reveals hyperthrombocytosis and hypercoagulation.
In a significant proportion of patients, abdominal syndrome is short-lived and goes away on its own in 2-3 days. Periods of severe pain may alternate with pain-free intervals lasting about 1-3 hours. This helps to distinguish abdominal syndrome from acute surgical diseases of the abdominal organs. Such differentiation is especially difficult in patients without skin and joint manifestations and with symptoms of peritoneal irritation. More often, abdominal syndrome imitates acute intestinal obstruction (intussusception), appendicitis, ovarian torsion and cysts, and perforation of an intestinal ulcer.
Comparative diagnostics can cause certain difficulties for the doctor - this is due to the fact that hemorrhagic vasculitis itself can cause all of the listed surgical diseases of the abdominal organs. For example, many cases of intussusception (invasion of one section of the intestine into another) and intestinal obstruction due to compression or closure of its lumen by a hematoma (especially in children under 2 years of age), intestinal necrosis and perforation (formation of a through defect), acute appendicitis have been described. and other complications requiring surgical intervention. The difficulties of differential diagnosis in such a situation lead to the fact that some patients with hemorrhagic vasculitis undergo unnecessary surgical interventions.
In adult patients, abdominal syndrome is observed less frequently and in most cases does not serve as a basis for diagnostic laparotomy, and is rarely complicated by intestinal obstruction and peritonitis (inflammation of the peritoneum). In old age
2. Reasons
The etiopathogenesis of Henoch-Schönlein hemorrhagic vasculitis (we note in passing that it is more correct to write the first of two German surnames with “I”) remains controversial, but by now more and more researchers are leaning towards the autoimmune hypothesis. The source of controversy is mainly the variety and heterogeneity of trigger factors that can provoke an attack by the immune system on the tissues of its own blood vessels. Such factors include respiratory infections, vaccination, viral diseases, taking certain medications (antibiotics, first-generation antipsychotics), allergic reactions, pregnancy, hypothermia, liver cirrhosis, oncological processes, etc.
Visit our Therapy page
When should you see a doctor?
If you begin to worry about the problems presented below, then urgently make an appointment with a specialist:
- A sudden rash in the form of crumbs in the areas of the arms, legs, hip area and face;
- Regular pain and discomfort in the joints, in particular this concerns the lower extremities of the body;
- Edema and swelling;
- The appearance of bruises;
- The presence of blood in the urine;
- A sharp rise in pressure.
Idiopathic thrombocytopenic purpura: from Werlhof to the present day
Spontaneous skin bleeding, known for more than 2500 years, was already called “purpura” in the Greek and Roman healing period. In 1735, Paul Werlhof (ITP is also called "Werlhof's disease") described a 16-year-old girl with epistaxis and mucosal bleeding that was controlled by the use of citric acid. He named this disease "Morbus Maculosus Haemorhhagicus". But noticeable progress in the treatment of patients with ITP was achieved later: in 1916 in Prague, Professor Schloffer removed the spleen from a woman with this disease. A significant increase in platelet counts followed surgery. And until now, splenectomy is one of the treatment options for patients with ITP. However, the most complete picture of this pathology, our understanding of the mechanisms of its occurrence and approaches to diagnosis and therapy have begun to emerge only recently.
Idiopathic thrombocytopenic purpura (ITP), or primary immune thrombocytopenia (ITP), is an acquired autoimmune disease characterized by isolated thrombocytopenia with a platelet count below 100x109/L. It can manifest itself as a hemorrhagic symptom of varying severity - from petechial skin hemorrhages to life-threatening bleeding. Both children and adults get sick. The etiology of ITP is unknown. That's why it's called "idiopathic". Among the triggering factors, the largest group includes infections, pregnancy, as well as vaccinations and stress.
It is known that the dominant mechanism for the development of thrombocytopenia in ITP is due to the production of autoantibodies to the membrane structures of platelets and their precursors - megakaryocytes, which lead to increased destruction of platelets by phagocytes, mainly in the spleen, less often in the liver, and insufficient production of platelets in the bone marrow. Patients with ITP develop primarily IgG autoantibodies against platelet surface glycoproteins GPIIb/IIIa or GPIb/IX. The process of forming an immune response to one’s own platelets is complex, multi-stage, and cyclical. B lymphocytes, T lymphocytes, NK cells, and macrophages take part in it. In addition to antibody formation, subpopulations of T-lymphocytes and the development of an imbalance in the T-cell component of the immune response play a major role in the pathogenesis of ITP. An association has been identified between ITP and some candidate genes, which also indicates the presence of a genetic predisposition to ITP.
Taking into account the concomitant pathology, the patient develops a certain phenotype of the disease. Thus, the pathogenesis of ITP is associated with profound disorders of the immune system. In this regard, idiopathic thrombocytopenic purpura has been renamed to primary immune thrombocytopenia of unknown etiology. Accordingly, in all other forms, immune thrombocytopenia with a known etiology will be a symptom of other autoimmune diseases - systemic lupus erythematosus (SLE), antiphospholipid syndrome (AFLS), rheumatoid arthritis (RA), etc.
Currently, the search continues for etiopathogenetic mechanisms of the development of this rare pathology, which could stratify patients into risk groups to individualize treatment tactics.
In the literature, ITP is described as a rare orphan disease. It must be said that in the medical world there is no single definition of this group of diseases. In some countries, orphan pathologies are identified depending on the number of sufferers, in others - on the availability of treatment methods, in others - only chronic, life-threatening ones are classified as rare diseases.
Russia has its own history of orphan diseases. Since the time in our country the definition of “orphan diseases” was legislatively adopted (Law No. 323 “On the fundamentals of protecting the health of citizens in the Russian Federation” dated November 21, 2011)1, namely: rare (orphan) diseases are diseases that are widespread no more than 10 cases of the disease per 100 thousand population, all oncohematological and many hematological diseases began to be considered orphan. As for ITP, according to Decree of the Government of the Russian Federation No. 403 of April 26, 2012, idiopathic thrombocytopenic purpura (D69.3) was included in the short list of life-threatening and chronic progressive rare (orphan) diseases leading to a reduction in the life expectancy of citizens or their disability. This short list also included such hematological conditions as paroxysmal nocturnal hemoglobinuria (Marchiafava-Miceli disease), aplastic anemia, hereditary metabolic diseases, hemolytic-uremic syndrome, etc.
All these legislative decisions led to the creation of a department - a hospital for orphan diseases (headed by Professor E.A. Lukina) at the Federal State Budgetary Institution "National Medical Research Center of Hematology" in 2012. The nosological range of pathologies dealt with by the department is very wide.
Medicines developed to treat rare diseases are also called orphan drugs and include a list of expensive drugs. Assigning orphan status to diseases and any drugs is a social and political issue in many countries, as well as in Russia. Government support for rare disease research has led to medical breakthroughs that could not have been achieved under the previously existing funding system.
The orphan disease status for ITP also opened up new opportunities for improving its diagnosis and treatment with modern methods that could not be achieved without it. We are talking primarily about two orphan expensive thrombopoietin receptor agonist (aTPO) drugs (romiplostim from Novartis and eltrombopag from Amgen). Medicines are provided at the expense of the budgets of the constituent entities of the Russian Federation.
Epidemiology
It should be recalled that in our country, the incidence of ITP at the population level was not studied until 2014. And there was not enough information to assess the course, effectiveness and safety of various treatment options for patients with ITP. To solve these problems, under the auspices of the National Hematological Society (chairman of the Supervisory Board of the NGO - chief freelance hematologist of the Russian Federation, director of the Federal State Budgetary Institution "National Medical Research Center for Hematology" of the Ministry of Health of Russia, academician of the Russian Academy of Sciences, Professor V.G. Savchenko) in early December 2012, " Registry of diseases of the blood system.”3 Since 2014, work has started in its subsection “ITP” - a multicenter prospective observational cohort study “Epidemiological and clinical characteristics of ITP in adults in Russia” (head: A.L. Melikyan) began.
According to the register of the National Hematological Society (NGO), the incidence of ITP in the adult population in the Russian Federation averages 2.0 (1.6‒3.6) per 100 thousand population per year. ITP has no geographical features. Men get sick 2–3 times less often than women. The largest proportion of patients (45.4%) were in the age group from 18 to 40 years, in the group from 41 to 60 years - 26.0% and over 60 years - 28.6%. Thus, among patients with ITP, 71.4% are of working age. The highest incidence of ITP was registered in women of fertile age 4. Our results are quite comparable with data from registers in other European countries.
Diagnostics
The main clinical manifestation of ITP is hemorrhagic syndrome, and the prognosis of the disease depends entirely on its severity. The risk of bleeding in patients with ITP is assessed by the platelet count in a peripheral blood test. According to the register, in 70.0% of cases, the number of platelets at the onset of the disease ranges from 3 to 30x109/l, among them, 35% have a critical level of platelets (from 3 to 10x109/l) with the risk of developing spontaneous alarming, life-threatening bleeding, which requires immediate treatment.
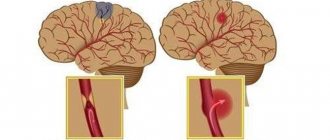
Hemorrhagic syndrome manifests itself in the form of: skin hemorrhages - 77% of cases; bleeding of oral mucosa – 39%; nosebleeds – 31%; menometrorrhagia – 15% (among women); gastrointestinal bleeding – 7%; hematuria – 4%; intracerebral bleeding - 0.9%, others - 1% (retinal hemorrhage, hemorrhoidal bleeding).4
Thus, about 1/3 of patients at the time of diagnosis have hemorrhagic manifestations corresponding to a severe form of ITP (grade 3–4 bleeding according to the WHO classification). ITP is not a genetic disease, but usually accompanies the patient throughout his life and is incurable. The course of the disease is further complicated by the fact that in 60–70% of patients after 12 months (chronic phase), the disease becomes chronic and relapsing again.
ITP is not a genetic disease, but usually accompanies the patient throughout his life and is incurable. The course of the disease is further complicated by the fact that in 60–70% of patients after 12 months (chronic phase), the disease becomes chronic, relapsing, and hemorrhagic syndrome reappears, requiring an anti-relapse course of therapy.
The diagnosis of ITP is a diagnosis of exclusion, i.e. To date, there is no specific test for the disease. Thrombocytopenia of various origins is recorded in a wide range of diseases of hematological, non-hematological and congenital nature, in which isolated thrombocytopenia may be the dominant clinical symptom for a long time. Therefore, to establish the true causes of thrombocytopenia, it is necessary to conduct an expanded diagnostic search at the onset of the disease.4
The initial approach to diagnosing the causes of thrombocytopenia is based on the patient's medical history (his underlying diseases and previous drug therapy), his objective physical examination and examination according to the protocol. The protocol for the differential diagnosis of thrombocytopenia that we developed is included in the National Clinical Guidelines for ITP.5 The most important thing is that all the proposed laboratory and instrumental studies exist in routine practice and are mandatory for all patients with suspected ITP.
After excluding other causes of thrombocytopenia, the diagnosis of ITP is made based on the following criteria:
- isolated thrombocytopenia less than 100.0x109/l, recorded in at least two consecutive blood tests;
- absence of morphological and functional platelet abnormalities;
- absence of pathology of lymphocytes, granulocytes and erythrocytes;
- normal hemoglobin, red blood cells and reticulocytes, if there was no significant blood loss;
- increased or normal number of megakaryocytes in the myelogram;
- normal size of the spleen.
It is important to keep in mind: to quickly relieve hemorrhagic syndrome, patients are often prescribed corticosteroids without examination according to the protocol, which blurs the true clinical picture of secondary immune thrombocytopenia and affects the true results of immunological tests. According to our department, in up to 15–20% of cases, during repeated examination according to the protocol, the diagnosis of ITP is replaced by another. The picture of the disease may change over time; therefore, it is necessary to constantly update data on the patient’s condition and carry out differential diagnosis at each stage of observation/therapy for ITP. Thus, it is very important to carry out a differential diagnosis between primary and secondary thrombocytopenia not only at the onset of the disease, but also during relapse of thrombocytopenia.
Establishing the true causes of thrombocytopenia is extremely important for choosing adequate therapy for such patients
In many cases, patients with primary and secondary ITP receive similar treatment. However, if ITP develops in the context of an underlying disease (eg, SLE, APS, HCV infection, HIV infection, or lymphoproliferative disorder), then treatment should be directed primarily at it.
It should be noted that our doctors’ awareness of this disease is at a fairly high level. Firstly, as stated above, ITP was described 275 years ago. Secondly, ITP is a fairly common rare disease. Every year, more than 300 patients diagnosed with or suspected of ITP are consulted in our department alone. And finally, for the first time in Russia (in 2014), National Clinical Recommendations (NCR) for the diagnosis and treatment of idiopathic thrombocytopenic purpura in adults, along with other nosologies, were developed on the initiative of the Russian Ministry of Health.4 These recommendations are constantly updated and are publicly available.4 The latest edition was published in 2021.
In 2021, updated clinical guidelines from the American Association of Hematology (ASH) and updated International Consensus on the Diagnosis and Treatment of Primary Immune Thrombocytopenia were released. We analyzed these recommendations and compared them with Russian clinical guidelines for ITP.
I want to say that we have not noticed any global changes: special attention is paid to correct diagnosis, and approaches to treatment are described in more detail.
In addition, the study of ITP is included in the educational and scientific plans of our Hematology Center for hematologists, residents, students in advanced training cycles of the Federal State Budgetary Institution “National Medical Research Center for Hematology” of the Ministry of Health of Russia and for specialists in other specialties, since there are hematological masks for various diseases. Our Center organizes on-the-job training for medical specialists on current issues in hematology for both hematologists and other specialists. All these programs are included in the system of continuing medical education. In the era of digitalization and distance learning technologies, holding webinars, master classes, and discussing specific clinical examples, the audience of students is significantly expanding, which contributes to the continuous professional growth of doctors.
Treatment
Splenectomy (SE), introduced into treatment practice at the beginning of the last century by Schloffer, is still one of the treatment options for patients with ITP. It is performed relatively frequently, but over the past three years the number of such interventions has decreased from 26 to 17%, while the proportion of patients receiving modern drugs (thrombopoietin receptor agonists, aTPO) has increased from 5.9 to 45.7%. The same trend is observed abroad, where the frequency of splenectomy is lower than in the Russian Federation.
Since 1951, corticosteroids have been used in the treatment of the disease; they still remain the first line of treatment for patients with newly diagnosed ITP in foreign and Russian protocols - with hemorrhagic syndrome and platelets less than 30‒50.0x109/l or in the absence of hemorrhagic syndrome with thrombocytopenia 9/ l.
According to our registry data, in 92.2% of cases, as a first line, patients receive treatment with corticosteroids, both in the form of standard treatment and pulse therapy, with an effectiveness of up to 70–80% with rapid relief of hemorrhagic syndrome and an increase in platelet counts above a safe level. However, after discontinuation of the drug, a relapse of the disease quickly occurs. Corticosteroids are effective, but they are associated with a large number of potential complications: diabetes mellitus; severe forms of arterial hypertension and arrhythmias; gastrointestinal ulcer, active infections; mental disorders. Therefore, repeated and frequent courses are undesirable. All clinical guidelines strictly limit the duration of treatment with corticosteroids to 3–4 weeks. Unfortunately, due to availability, corticosteroids are also prescribed in subsequent lines of therapy.
In 1980, at the University Children's Hospital in Bern, a 12-year-old boy with acute ITP and immunodeficiency was treated with intravenous immunoglobulin (IVIG), which resulted in a marked increase in platelet count within 24 hours. Since then, IVIG has been widely and successfully used in both first and subsequent lines of therapy as an “ambulance” in emergency and life-threatening situations and is an absolutely invaluable tool for the treatment of pregnant women with ITP. IVIG as a first-line therapy is effective in 80% of cases, the hemostatic effect occurs on days 1–2, and the duration of response is 1–4 weeks. Thus, in fact, after first line therapy, almost all patients are candidates for second line therapy.
To systematize the procedure for prescribing treatment options, the international working group for the study of ITP identified 3 stages of the disease:
- newly diagnosed with a duration of up to 3 months from the moment of diagnosis;
- persistent with a duration of 3–12 months;
- chronic with a duration of more than 12 months.
And the sequence of prescribing therapy for ITP, developed on the basis of many years of clinical experience, is called lines of therapy, which generally correspond to the stages of the disease.
At the end of 2000, without a doubt, a new era of treatment for ITP begins: with modern-generation drugs - thrombocytopoiesis stimulants, thrombopoietin receptor agonists, and TPOs - romiplostim (Novartis) and eltrombopag (Amgen). In 2009, they were approved in Russia as orphan drugs for adults with refractory chronic ITP with or without splenectomy. In 2015, both drugs were included in the List of vital and essential drugs for medical use, approved by order of the Government of the Russian Federation. The use of aTPO (these data are based on evidence-based medicine and are supported by several solid and very high-quality prospective control studies) is effective both before and after splenectomy. With the advent of these drugs, the prognosis of the disease has improved, since they prevent the development of severe side effects of treatment and allow achieving an 80% level of immediate effect.1,6
I believe (like many of my colleagues) that their important features are organ-preserving and corticosteroid-restraining effects.
Another drug for the treatment of ITP, rituximab, has recently appeared in clinical practice, which was developed for the treatment of hematological malignancies. Rituximab is currently used to treat patients with ITP who are refractory to other treatments. Its use in chronic ITP is based on the removal of autoreactive B lymphocytes. Rituximab is included as 3rd line therapy. There is approximately a 60% chance of obtaining a primary response. But in Russia it is not registered for the treatment of ITP, so the decision is made individually by a medical commission.
In 2021, the US Food and Drug Administration (FDA) approved a new oral drug, the selective small molecule splenic tyrosine kinase inhibitor, fostamatinib, for medical use in patients with refractory ITP. And in 2021, a bioavailable small molecule thrombopoietin receptor agonist, avatrombopag, for the treatment of adult patients with chronic ITP who have had an insufficient response to previous therapy. Both drugs are not registered in Russia for the treatment of ITP. This is perspective.
If various treatment options are unsuccessful in subsequent lines of therapy, it is recommended to use a non-implementing method or carry out complex therapy using immunosuppressants.
As a rule, modern methods of therapy still make it possible to achieve remission of varying durations or states of clinical compensation. But clear prognostic criteria for the course of the disease, response to therapy and disease outcomes have not yet been developed - due to the nature and unpredictable course of the disease.
When starting treatment for chronic, recurrent ITP, it is necessary to remember that the choice of therapy should be aimed at stopping bleeding of any location, improving the patient’s quality of life, and not at normalizing the platelet count at any cost.
In clinical practice, it is important to remember that therapy should always be selected individually for a particular patient, taking into account his age, comorbidity, concomitant pathology, and also taking into account the patient’s preferences. But our practice often collides with the objective realities of life.
According to E.Yu. Krasilnikova (head of the project office “Rare (orphan) diseases” of the National Research Institute of Public Health named after N.A. Semashko), when preparing the Annual Bulletin on rare (orphan) diseases9, information was received from 76 regions of the Russian Federation in which, as of January 1, 2021, There were 3,860 patients with ITP (873 of them were children), 2,069 people needed drug therapy (468 of them were children), 1,606 patients (of which 446 were children) received drug therapy. That is, pathogenetic treatment was provided to 42% of patients included in the Federal Register of Persons Suffering from Rare Life-Threatening Diseases.8
In fact, more than half of patients do not receive the intended orphan treatment. The quality of medical care, which includes diagnosis, determination of treatment tactics, correction and control over these indicators, became possible thanks to the development of a patient routing scheme, which represents the patient’s path from diagnosis to provision of necessary medications, that is, from the attending physician to the inclusion of patients in federal register list.
Our experience with regional hematologists is that they are all knowledgeable and respectful of patient routing pathways for orphan drugs. In difficult situations, they turn to federal centers to obtain a decision from a medical commission on expensive drugs. Providing only 42% of patients with ITP in the regions with modern, expensive orphan drugs is mainly due to insufficient funding in a number of regions. And this also has its explanation. The number of patients requiring expensive orphan drugs is increasing due to improved diagnostics. The only way out is to include ITP in the federal program for financing high-cost nosologies.
Thus, idiopathic thrombocytopenic purpura (ITP) is a rare (orphan) chronic, relapsing disease that significantly worsens the health and quality of life of patients as assessed by physical, social functioning, and mental state. Bleeding causes fear, anxiety and depression in them due to the short-term effect of the therapy and side effects of drugs during long-term treatment with corticosteroids and immunosuppressants.
ITP cannot be completely cured, but it can be effectively controlled. Modern drugs (thrombopoietin receptor agonists) that have appeared in recent years, with adequate choice of dose and control of the course of the disease, can quickly stop hemorrhagic syndrome, achieve remission of varying durations or a state of clinical compensation, prevent the development of severe side effects of treatment, improve the prognosis of the disease, which, naturally, not only increases the life expectancy of patients with orphan diseases, but also its quality. Therefore, it is very important to include them in the therapy of all patients who need it. Today, this equal accessibility is possible only with the inclusion of ITP in the federal program for financing high-cost nosologies.
Literature
- “On the fundamentals of protecting the health of citizens in the Russian Federation” No. 323-FZ dated November 21, 2011. RG, federal issue No. 263 (5639) (dated November 23, 2011). https://rg.ru/2011/11/23/zdorovie-dok.html
- Decree of the Government of the Russian Federation No. 403 of April 26, 2012 “On the procedure for maintaining the Federal Register of persons suffering from life-threatening and chronic progressive rare (orphan) diseases leading to a reduction in the life expectancy of citizens or their disability, and its regional segment.” May 2, 2012. https://www.garant.ru/products/ipo/prime/doc/70068888/
- Chernikov M.V., Kulikov S.M., M.A. Rusinov M.A. et al. Multinosological register of diseases of the blood system. Composition, structure, results of trial operation // Hematology and transfusiology. T. 59, No. 1, 2014, p. 30. Melikyan A.L., Egorova E.K., Pustovaya E.I., Kolosheinova T.I., Volodicheva E.M., Kaporskaya T.S., Ilyasov R.K., Shelekhova T.V., Fedorova N.A., Zotova I.I., Sycheva T.M., Kontievsky I.N., Shestopalova I.A., Kurkina N.V., Syrtseva E.B., Tarasenko E.V. Interim results of an epidemiological study of idiopathic thrombocytopenic purpura in adults in the Russian Federation // Hematology and Transfusiology. 2021. T 64. No. 4. P. 436‒446.
- Melikyan A.L., Pustovaya E.I., Egorova E.K., Kalinina M.V., Kolosheinova T.I., Subortseva I.N., Gilyazitdinova E.A., Dvirnyk V.N. Differential diagnosis of thrombocytopenia // Oncohematology. 2017;12(1):78‒87. https://doi.org/10.17650/1818-8346-2017-12-1-78-87
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2021 guidelines for immune thrombocytopenia. 2019;3(23):3829‒3866. doi:10.1182/bloodadvances.201900096
- Melikyan A.L., Pustovaya E.I., Egorova E.K., Kalinina M.V., Kolosheinova T.I., Subortseva I.N., Gilyazitdinova E.A., Dvirnyk V.N. Differential diagnosis of thrombocytopenia // Oncohematology. 2017;12(1):78‒87. https://doi.org/10.17650/1818-8346-2017-12-1-78-87
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780‒3817. doi:10.1182/bloodadvances.2019000812
- Annual bulletin of the expert council on rare (orphan) diseases. https://komitet2-.km.duma.gov.ru/upload/site21/Byulleten_po_redkim_zabolevaniyam_2020.pdf
- Clinical guidelines “Idiopathic thrombocytopenic purpura (ITP) in adults” (approved by the Ministry of Health of Russia), 2021. https://npngo.ru/uploads/media_document/283/5eb37419-9276-4e9a-b075-0e26a788f623.pdf
1.General information
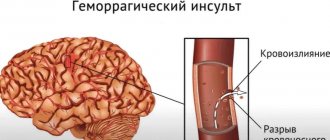
Bleeding inflammation of the vascular walls is a fairly common disease, also known as Henoch-Schönlein disease, Henoch-Schönlein purpura or anaphylactic vasculitis (in Western literature and ICD-10), hemorrhagic vasculitis (in domestic medicine), rheumatic or allergic purpura, - which manifests mainly in childhood or early puberty.
The incidence is estimated at 0.2-0.25% among people in this age category. Hemorrhagic vasculitis is considered one of the most common types of pathology of vascular walls. Small vessels of the skin and internal organs are affected; joints, abdominal organs, and kidneys may be involved. The disease was first described by I.L. Schönlein (1837) and then studied in more detail by E.N. Henoch (1874), which served as the basis for the name of the disease.
A must read! Help with treatment and hospitalization!
Why does rheumatic purpura appear?
Hemorrhagic vasculitis, also called Henoch-Schönlein disease, is a type of inflammation of small areas of the vascular system.
A similar process can occur in the human body due to failures in the stable functioning of the immune system. The main sources of disease development include:
- Acute and chronic diseases caused by bacterial or viral infection (for example: acute respiratory viral infections, staphylococci, herpes rashes, etc.)$
- Hereditary predisposition;
- Poisoning of the body with biological poisons and chemicals;
- Prolonged exposure to frost or heat;
- Insect bites;
- Personal reaction to the introduction of special types of vaccine preparations;
- Sunburn of the skin.