Compound
Terbinafine tablets contain the active substance terbinafine hydrochloride , as well as additional components: MCC, lactose monohydrate, potato starch, talc, primellose, aerosil, magnesium stearate.
Terbinafine cream contains the active component terbinafine hydrochloride , as well as additional components: benzyl alcohol, stearic acid, distilled glycerol, petrolatum, emulsifier, water, triethanolamine.
The ointment contains the active component terbinafine hydrochloride , as well as additional components: methyl parahydroxybenzoate, carbomer , petroleum jelly, polysorbate, sodium hydroxide, propylene glycol, water.
Terbinafine spray contains the active component terbinafine hydrochloride , as well as additional components: macrogol 400 , water, propylene glycol, ethyl alcohol.
pharmachologic effect
Terbinafine is a fungicidal, antifungal drug. Demonstrates activity against almost all types of fungi that can infect the human body. In small concentrations it exhibits a fungicidal effect against molds, dermatophytes, and some types of dimorphic fungi. Both fungicidal and fungistatic effects are possible on yeast fungi.
Its therapeutic effect is determined by its destructive effect on the fungal cell membrane, as well as due to the specific inhibition of squalene epoxidase (an enzyme important for the normal function of the fungal cell membrane).
Under the influence of Terbinafine, the production of ergosterol , due to the lack of which the amount of squalene in the fungal cell increases. As a result, all enzyme systems are inactivated, and the cell dies.
The active component does not affect the cytochrome P450 system, therefore, does not affect the metabolism of hormones or other drugs.
Terbinafine cream for external use 1% 15g
Compound
Active substance: terbinafine hydrochloride - 10 mg.
Pharmacokinetics
When applied externally, the drug quickly penetrates the dermal layer of the skin and accumulates in the lipophilic stratum corneum. When applied externally, absorption is less than 5% of the drug dose and has a slight systemic effect.
Indications for use
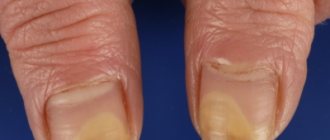
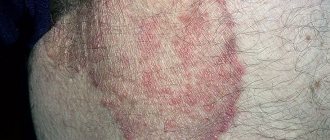
Prevention and treatment of fungal skin infections, including mycoses of the feet (“fungus” of the foot), keratinization, cracks, itching and peeling of the skin caused by “fungus” of the foot; inguinal athlete's foot (tinea cruris), fungal infections of the smooth skin of the body (tinea corporis) caused by dermatophytes such as Trychophyton (including Trychophyton rubrum, Trychophyton mentagrophytes, Trychophyton verrucosum, Trychophyton violaceum), Microsporum canis and Epidermophyton floccosum.
Yeast infections of the skin, mainly those caused by the genus Candida (eg Candida albicans'), particularly diaper rash.
Versicolor (Pityriasis versicolor), caused by Pityrosporum orbiculare (also known as Malassezia furfur).
Contraindications
Hypersensitivity to terbinafine, children under 12 years of age.
Carefully
Renal and/or liver failure, alcoholism, suppression of bone marrow hematopoiesis, tumors, metabolic diseases, occlusive vascular diseases of the extremities.
Directions for use and doses
Apply externally 1-2 times/day.
Adults and children over 12 years of age: Before applying the cream, the affected areas must be cleaned and dried. The cream is applied 1 or 2 times a day in a thin layer to the affected skin and adjacent areas and rubbed in lightly. For infections accompanied by diaper rash (under the mammary glands, in the spaces between the fingers, between the buttocks, in the groin area), the places where the cream is applied can be covered with gauze, especially at night.
Duration of treatment and frequency of use of the drug
Dermatomycosis of smooth skin and inguinal dermatomycosis (including dermatomycosis of the trunk, dermatomycosis of the legs): 1 week 2 times a day (morning and evening).
Ringworm of the feet: 1 week 2 times a day between the toes (morning and evening), 2 weeks 1 time a day (upper and lateral parts of the foot).
Fungal skin infections caused by yeast (cutaneous candidiasis): 1 week, 1 time per day.
Tinea versicolor: 2 weeks, 1 time per day.
If after 1-2 weeks of treatment there are no signs of improvement, the diagnosis should be verified.
Elderly patients (over 65 years old)
In elderly people, no dose adjustment is required.
Storage conditions
In a place protected from light at a temperature not exceeding 25°C.
Keep out of the reach of children.
Best before date
2 years. Do not use after the expiration date stated on the packaging.
special instructions
Terbinafine is for external use only.
A decrease in the severity of clinical manifestations is usually observed in the first days of terbinafine use. In case of irregular use or premature cessation, there may be a risk of recurrence of infection.
During the period of use of terbinafine, it is necessary to observe general hygiene rules to prevent reinfection (through underwear, shoes).
If terbinafine accidentally gets into your eyes, rinse them immediately with running water, and if persistent irritation develops, you should consult a doctor.
If allergic reactions develop, the use of terbinafine should be discontinued.
Description
Antifungal drug for external use.
Use in children
Use from 12 years of age.
Pharmacodynamics
An antifungal agent for external use with a wide spectrum of antifungal activity. In small concentrations, terbinfine has a fungicidal effect on dermatophytes (Trychophyton Rubrum, Trichophyton Mentagrophytes, Trichophyton Verrucosum, Trichophyton Violaceum, Trichophyton Tonsuras, Micross Porum Canis, Epidermophyton Floccosum), mold (for example, Scopulariopsis Breviculis) and certain dimorphous mushrooms (Pityrosporum Orbiculare). Activity against yeast fungi, mainly Candida albicans, depending on their type, can be fungicidal or fungistatic.
Terbinafine specifically alters the early stage of sterol biosynthesis occurring in fungi. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes the death of the fungal cell. Terbinafine acts by inhibiting the enzyme squalene epoxidase located on the cell membrane of the fungus.
Terbinafine does not affect the isoenzymes of the cytochrome P450 system in humans and, accordingly, the metabolism of hormones or other drugs.
Side effects
Classification of the frequency of side effects (WHO): very often (≥1/10); often (≥ 1/100, < 1/10); uncommon (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (< 1/10000), including isolated reports.
From the immune system:
Individual messages:
hypersensitivity reactions (rash).
From the side of the organ of vision:
Rarely:
eye irritation.
From the skin:
Often:
peeling of the skin, itching.
Infrequently:
skin damage, crusting, skin lesions, pigmentation disorders, erythema, burning sensation of the skin.
Rarely:
feeling of dry skin, contact dermatitis, eczema.
Individual messages:
rash.
Local reactions:
Infrequently:
pain, pain at the application site, irritation at the application site.
Rarely:
exacerbation of symptoms of the disease.
In areas where the drug is applied, itching, peeling of the skin, pain, irritation, changes in skin pigmentation, burning, erythema, and crusts may be observed. These minor symptoms should be distinguished from hypersensitivity reactions, such as rash, which occur in rare cases and require discontinuation of therapy. In rare cases, the course of a fungal infection may worsen.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Use during pregnancy and breastfeeding
Use during pregnancy is possible if the potential benefit to the mother outweighs the possible risk to the fetus/child.
Terbinafine passes into breast milk in very small quantities.
Terbinafine should not be applied to the mammary glands during breastfeeding.
Interaction
There are no known drug interactions with terbinafine cream.
Overdose
There have been no reports of cases of drug overdose with topical use.
In case of intentional or accidental ingestion of Terbinafine cream, nausea, headache, epigastric pain and dizziness can be expected.
Treatment: gastric lavage, intake of activated charcoal, and, if necessary, symptomatic maintenance therapy.
Impact on the ability to drive vehicles and operate machinery
Does not affect.
Pharmacokinetics and pharmacodynamics
When taking the medicine orally, the active substance is rapidly absorbed from the digestive tract. Its partial metabolism occurs in the liver, as a result, its bioavailability decreases to 40%. The bioavailability of the drug is only slightly affected by food intake, so there is no need to adjust the dose.
The highest concentration of the drug in the blood is observed 2 hours after the 250 mg tablet was taken. 99% of the active substance in the blood binds to plasma proteins.
The drug is noted to be onycho- and epidermotropic, that is, its largest amount (concentrations optimal for a therapeutic effect) accumulates in the hair, skin, nails, and also in the subcutaneous tissue.
In the body, terbinafine hydrochloride is biotransformed into metabolites that do not demonstrate antifungal activity. Most of them are excreted in the urine. The half-life is 17 hours.
There is no accumulation of Terbinafine when taken. Its effectiveness is the same for all patients, regardless of the person’s age. In the presence of pathological changes in the liver and kidneys, the biotransformation of the drug may slow down. As a result, its concentration in biological fluids increases and the period of circulation of the drug in the blood increases.
When using Terbinafine topically, no more than 5% of the active component enters the blood.
Indications for use
The instructions for the use of Terbinafine indicate that all forms of the drug are used for diseases that were caused by yeast-like , molds , and dermatophytes .
The drug in tablets is indicated for use in diseases of fungal origin that were caused by dermatophytes of the genus Trichophyton (T. Mentagrophytes, T. verrucosum, T. Violaceum, T. Rubrum, T. tonsurans), Microsporum canis, Epidermophyton floccosum, as well as fungi of the genus Candida. Tablets are prescribed for trichophytosis , microsporia , onychomycosis , epidermophytosis , candidiasis .
As a rule, Terbinafine tablets are prescribed for widespread and severe symptoms. At the same time, treating lichen versicolor with tablets is ineffective.
What the cream, ointment and spray are for, and whether these local remedies should be used, should be determined by a specialist.
As a rule, the cream should be used for fungal diseases caused by Candida, Microsporum canis, Trichophyton, Pityriasis, Epidermophyton floccosum fungi.
Also, ointment and cream are used for candidiasis, skin lesions caused by dermatophytes, and lichen versicolor.
Instructions
The ointment is used strictly externally. Recommended for use by adults and children over 12 years of age. Before applying the product, it is necessary to remove all contaminants from the surface of the skin and dry it. A small amount of cream is rubbed into the lesion, covering a small area beyond the edges of the inflammation. Repeat the procedure in the morning and evening.
The course of treatment depends on the degree of damage and the type of fungal infection:
- smooth skin, including areas on the back, abdomen and lower legs, require 1 week of treatment;
- foot fungus goes away within 8-9 days of using Terbinafine ointment;
- treatment of candidiasis can last up to 2 weeks;
- It also takes 10 to 14 days to get rid of tinea versicolor.
If the use of the ointment does not give the desired effect over time, or during treatment there is a clear progression of the disease, accompanied by growth of the affected area, it is necessary to contact a specialist to prescribe systemic antifungal agents or to change the treatment regimen with local agents.
Contraindications
The use of medication in tablet form is contraindicated for the following diseases and conditions:
- liver disease in chronic or active form;
- chronic renal failure (creatinine clearance less than 50 ml/min);
- the patient’s age is up to three years and his weight is up to 20 kg;
- lactose intolerance, lactase deficiency, glucose-galactose malabsorption;
- lactation;
- high sensitivity to the components of the product.
Caution Terbinafine tablets should be prescribed to people with chronic renal failure (with glomerular filtration more than 50 ml/min based on the Rehberg test), hematopoietic disorders, alcoholism , endocrine diseases, psoriasis , vasoconstriction of the extremities, tumors, systemic and cutaneous lupus erythematosus.
When taking Terbinafine, the condition of the liver and kidneys should be closely monitored. Terbinafine or Terbinafine Teva tablets should be discontinued immediately if the following symptoms occur:
- abdominal pain;
- nausea;
- loss of appetite;
- jaundice;
- weakness;
- darkening of urine;
- light cal.
Forms of the drug for topical use should not be used in case of hypersensitivity or allergies.
Relative contraindications to the use of such drugs are:
- tumors;
- alcoholism;
- liver and kidney failure;
- endocrine diseases;
- narrowing of the lumen of blood vessels;
- hematopoietic disorder;
- The patient's age is up to 12 years.
Terbinafine solution for external use 10 mg/1 ml 25 ml No. 1
Name
Terbinafine solution dnar.approx. 10 mg 1 ml in bottle 25 ml in pack No. 1
Description
clear colorless or yellowish liquid
Main active ingredient
Terbinafine
Release form
solution
Dosage
25ml
Indications for use
fungal infections of the skin caused by dermatophytes such as Trichophyton (including T. rubrum, T. mentagrophytes, T. verrucosum, T. violaceum), Microsporum canis and Epidermopyton floccosum; tinea versicolor, caused by Pityrosporum orbiculare (also known as Malassezia furfur).
Directions for use and doses
The medicine is intended for use in adults and children over 12 years of age. Terbinafine is used once or twice a day, depending on the indications. Before using the medicine, it is necessary to thoroughly clean and dry the affected areas. The solution is applied to the affected areas and adjacent intact areas of skin in an amount sufficient to thoroughly moisturize them, using cotton swabs if necessary to optimize this process. The area where the drug is applied is covered with a gauze bandage. Duration of treatment and frequency of use of the drug: dermatomycosis of the trunk, legs - 1 time per day for 1 week; dermatomycosis of the feet - once a day for 1 week; lichen versicolor - 2 times a day for 1 week. The effectiveness and safety of terbinafine solution for the treatment of moccasin-type foot mycoses, as well as severe foot mycoses with concomitant onychomycosis, have not been studied. A decrease in the severity of clinical manifestations is noted in the first days of treatment. In case of irregular treatment or its premature termination, there is a risk of recurrence of infection. If after a week of treatment there are no signs of improvement, the diagnosis should be verified.
Use during pregnancy and lactation
Due to the fact that there is no data on the safety of terbinafine during pregnancy and lactation, the drug should be prescribed only in cases where the expected positive effect in the mother outweighs the potential risk to the fetus. It is known that terbinafine is excreted in breast milk, so the drug should not be prescribed during breastfeeding.
Precautionary measures
Terbinafine solution is for external use only. The solution should not be applied to the face. Contact with eyes should be avoided as it may cause irritation. If the drug accidentally gets into your eyes, they should be rinsed with running water. Caution should be exercised when applying Terbinafine to damaged areas of the skin, as the drug contains alcohol, which can cause irritation. The propylene glycol contained in the drug may cause skin irritation. When treating with Terbinafine, general hygiene rules should be observed to prevent the possibility of re-infection (through underwear, shoes, etc.).
Interaction with other drugs
The interaction of terbinafine topical solution with other drugs is unknown.
Contraindications
Hypersensitivity to terbinafine or to any of the excipients included in the drug. With caution Renal/liver failure, alcoholism, suppression of bone marrow hematopoiesis, tumors, metabolic diseases, occlusive vascular diseases of the extremities. Use in children The drug should not be used in children under 5 years of age due to the lack of clinical data. There is not enough data on the safety and effectiveness of the drug in children under 12 years of age. Elderly patients No dose adjustment is required. Side effects do not differ from those observed in patients of other age groups.
Compound
1 ml of solution contains 10 mg of terbinafine hydrochloride as an active ingredient. Excipients: ethyl alcohol, purified water, macrogol 400, propylene glycol.
Overdose
There are no data on overdose with topical application of Terbinafine. If the solution is accidentally taken orally, you can expect the development of side effects such as headache, nausea, epigastric pain and dizziness. In such cases, gastric lavage and/or supportive symptomatic treatment may be necessary. You should also take into account the alcohol content of the drug (23.5%). Treatment: activated carbon, if necessary, symptomatic supportive therapy in a hospital setting.
Side effect
Redness, itching or burning sensation, peeling of the skin, pain or irritation may appear at the sites where the solution is applied, but discontinuation of treatment due to these phenomena is rarely required. These less serious side effects should be distinguished from rare allergic reactions (for example, generalized rash and/or redness, urticaria, angioedema, repeated allergic reaction), the development of which requires cessation of treatment. In rare cases, a fungal infection may worsen. If the listed adverse reactions occur, as well as reactions not listed in the package insert, you should consult a doctor.
Storage conditions
Store in a place protected from light at a temperature not exceeding 25°C. Keep out of the reach of children.
Side effects
When taking pills, patients may experience the following side effects:
- feeling of pain and weakness in the epigastric region;
- loss of appetite;
- cholestasis;
- taste disturbance;
- allergic reactions;
- nausea;
- diarrhea;
- decreased levels of platelets and neutrophils in the blood.
When applied topically, itching, burning and hyperemia may occur in the areas where the product was applied. Allergic manifestations may develop in rare cases.
Instructions for use of Terbinafine (Method and dosage)
Terbinafine tablets, instructions for use
The duration of taking the tablets should be determined by the doctor, taking into account the severity of the disease. Children should take the product after meals, this should be done once a day. When determining a single dose of medication, it is necessary to take into account the child’s body weight.
Children weighing less than 20 kg receive 62.5 mg of the drug, children weighing from 20 to 40 kg - 125 mg, children weighing over 40 kg - 250 mg.
Adult patients receive 250 mg Terbinafine Teva or Terbinafine tablets once daily or 125 mg twice daily.
The duration of treatment depends on the disease. For tinea pedis, you need to take the tablets for 2 to 6 weeks.
For candidiasis of the skin, dermatomycosis of the extremities, trunk, legs, treatment lasts from 2 to 4 weeks.
For infections of the scalp, treatment lasts 4 weeks.
For onychomycosis, effective treatment of the disease is possible if the drug is taken for 6 to 12 weeks. Sometimes, if the patient has a reduced rate of nail growth, treatment may take longer. The effect will be observed several months after completion of the course of treatment.
Terbinafine ointment, instructions for use
Ointment or cream is applied to the affected areas 1-2 times a day. Before applying the product, you need to very thoroughly clean and dry the affected skin. Apply a thin layer of the product to the affected areas and surrounding areas and rub in lightly. If during the development of the infection the patient experiences diaper rash , these areas can be covered with gauze after applying the product. It is advisable to do this if the ointment or cream is applied at night.
The duration of treatment depends on the disease. For candidiasis of the skin, dermatomycosis of the extremities, torso, and legs, the product should be applied for 1-2 weeks.
When treating lichen versicolor – 2 weeks.
Treatment of tinea pedis lasts 2-4 weeks.
For mycosis of the nail plates, the product is applied for 3-6 months.
As a rule, clinical manifestations decrease after the first days of using the medicine. It should be borne in mind that if the product is not applied regularly or therapy is stopped prematurely, the infection may recur. Provided that after two weeks of regular use of Terbinafine the manifestations of the disease do not decrease, you should consult a doctor and clarify the diagnosis.
The spray is used externally, it should be used 1-2 times a day.
Modern possibilities of using terbinafine for the treatment of fungal diseases
The incidence rate increases significantly in patients in older age groups, regardless of gender. According to foreign researchers, onychomycosis affects from 2 to 18.5% of the total population of the planet, and in the age group of 70 years and older, 50% of the world’s population is affected by this disease [1,8]. According to various authors, fungal infections of the feet are observed in a third of all European residents [18,20]. As a rule, the development of onychomycosis is preceded by mycosis of the feet - according to our data, only 30% of patients had isolated onychomycosis, in the remaining 70% of patients it was combined with mycosis, which began before the fungal infection of the nails [2]. Thus, treatment of mycosis of the feet is the prevention of the development of onychomycosis. Very often, dermatologists are faced with insufficient awareness of patients - the latter do not know about the presence of a fungal infection, even seeing manifestations on the skin in the form of peeling, intertrigo, hyperkeratosis, and attribute this to physiological characteristics or age-related changes. On the other hand, some patients, seeing changes in the nail plates, regard this as onychomycosis and begin to treat it themselves with all available means. In the presence of mycosis of smooth skin without affecting the nails and involving the hair in the process, a cure can be achieved using only topical antimycotics. One of the most active antimycotics is terbinafine. It belongs to allylamine derivatives. In terms of the breadth of the spectrum of antifungal action, this drug surpasses all other antifungal drugs: polyene antibiotics, azoles (imidazole derivatives - clotrimazole, ketoconazole, etc., as well as first generation triazoles - itraconazole, fluconazole, etc.) and echinocandins. In recent years, many studies have been conducted confirming the high effectiveness and safety of terbinafine (Lamisil) in the treatment of fungal infections. The undoubted advantages of this drug include a large number of its forms for external use - cream, dermgel, spray and film-forming solution for the treatment of types of mycosis. Terbinafine in the form of a cream is ideal for squamous and squamous-hyperkeratotic forms. In the first case, it is enough to apply the cream to the affected area once a day. within 7 days [15]. For the treatment of hyperkeratotic forms of mycosis, use the drug 2 times a day. within 2 weeks. makes it possible to achieve clinical and mycological cure. In a study conducted by Yu.N. Perlamutrov and K.B. Olkhovskaya, it has been shown that complete mycological cure is achieved with the use of terbinafine 1 time per day. within 2 weeks. occurred in 80% of patients, and 2 times a day. – in 93%. At the same time, by the end of the course of treatment, the disappearance of peeling, itching and healing of deep and superficial cracks was observed in patients in both groups, but when using the cream 2 times a day. these symptoms resolved more quickly [4]. For intertriginous and dyshidrotic forms, it is appropriate to prescribe dermgel or spray - they have a pronounced antipruritic and drying effect. It is also convenient to prescribe the spray in the presence of lesions on smooth skin (lichen versicolor, microsporia) in the folds and on the scalp. So, according to A.A. Haldina et al., when applying both spray and dermgel to lesions in large folds, etiological cure was observed in 100% of cases, regardless of the nature of mycosis (Tr. rubrum, Ep. floccosum, C. albicans). When analyzing the dynamics of regression of skin symptoms, it was revealed that when using the spray, the regression of clinical symptoms is somewhat ahead of that when using dermgel, this is especially noticeable with the regression of itching and maceration [7]. There is a high effectiveness of using spray and dermgel for the treatment of patients with pityriasis versicolor. According to L.P. Kotrekhova et al., the effectiveness of dermgel and spray therapy was 94.8 and 93.8%, respectively. At the same time, patients note the ease of use and good organoleptic properties of the drug, such as the absence of greasiness and oily sheen of the skin. The hygroscopic properties of the drug made it possible to apply it to skin folds even in obese patients [3]. A new, and certainly promising form is a film-forming solution, which is applied to the skin of the feet once. N.N. Potekaev et al. treated 20 patients with various forms of mycosis of the feet with Lamisil Uno. Clinical and mycological cure occurred in 80% of patients by the 10th day of observation. The drug was ineffective in the squamous-hyperkeratotic form. For erased, squamous and intertriginous forms, the effectiveness is 100%. I would especially like to note that single use of the drug is very convenient for patients [5]. A randomized, double-blind, placebo-controlled study was conducted on the effectiveness of a single application of a 1% film-forming solution of terbinafine in 324 patients with mycosis of the feet. The result was assessed after 6 weeks. after using the drug. The study found negative culture, microscopy, and mycological cure rates of 91%, 81%, and 78%, respectively, while the placebo response rate was 17% [11]. Another very important point is the possibility of curing mycosis in a short time - with squamous and intertriginous forms of the lesion, the disease is cured in 1 week, while azole drugs must be used for 4 weeks. [17,18]. A longer course of therapy often leads to non-compliance with the treatment regimen; patients stop using the drug after visible improvements and are not treated further, which leads to relapse of the disease. In some cases (with damage to the nail plates, a common skin process, hair involvement), local antifungal therapy is not effective enough, and there is a need to prescribe a systemic drug, the most effective and safe of which is terbinafine [19]. Terbinafine has a fungicidal effect on dermatophytes, molds and some dimorphic fungi; on yeast fungi, depending on their type, it has a fungistatic or fungicidal effect [13,16]. The pronounced antimycotic effect of terbinafine also manifests itself in vivo, which is facilitated by its pharmacokinetics. After oral administration, the maximum concentration of terbinafine in the blood is reached within 2 hours, the drug is almost completely bound to plasma proteins. Due to its lipophilicity, terbinafine quickly diffuses through the dermis and accumulates in the stratum corneum and nail plates, and is also excreted with sebum, resulting in high concentrations of the drug in hair follicles and hair. Stable concentrations of terbinafine in tissues are achieved 10–14 days after the start of oral administration, the half-life of its elimination varies from 24 to 156 days, as a result of which the drug remains in the nail plates for a long time after stopping its use [8,10]. It is known that fungal infections of the scalp can be successfully treated with terbinafine. To confirm this, 3 groups of children aged 2 to 14 years with a confirmed diagnosis of trichophytosis were treated with griseofulvin for a month and terbinafine for 2 weeks. and 1 month It was shown that the effectiveness of therapy was approximately the same and amounted to 100% with griseofulvin, 95% with a 2-week course of terbinafine and 94.1% with a monthly dose [12]. A study was conducted on the effectiveness of oral terbinafine for the treatment of a hyperkeratotic form of mycosis of the feet with a torpid course. The drug at a dose of 125 mg was prescribed daily for 1 month. It was found that the maximum concentration of the drug (247.8 ng) in the stratum corneum of the epidermis was determined by the end of 1 week. treatment, which is 50 times the minimum inhibitory concentration for dermatophytes. After 6 weeks the concentration of the drug decreased to 50.73 ng, and after 8 weeks. terbinafine was no longer detectable in the skin. The effectiveness of therapy was 95% [14]. The low potential for drug interactions with other drugs is also important, because Onychomycosis often affects older people who are forced to take a large number of different medications for other diseases [13]. Thus, based on the analysis of literature data, we can conclude that terbinafine (Lamisil) is a highly effective drug for the treatment of fungal infections, has a large number of different forms, which significantly expand the possibilities of antifungal therapy. Literature 1. Malcolm B. Athlete's foot // Attending physician. 1998. No. 6. 2. Vasenova V.Yu. Immunopathogenesis, morphofunctional characteristics, clinical picture, complex therapy and prevention of onychomycosis: Abstract of thesis. ... doc. honey. Sci. M., 2008. 3. Kotrekhova L.P., Vasilyeva N.V., Raznatovsky K.I., Piotrovskaya I.V. Clinical efficacy and safety of terbinafine (Lamisil spray, Lamisil dermgel) in the treatment of pityriasis versicolor // Clinical dermatology and venereology. 2007. No. 3. P. 35–38. 4. Perlamutrov Yu.N., Olkhovskaya K.B. Optimization of therapy for mycoses of the feet in women using 1% Lamisil cream // Clinical dermatology and venereology. 2006. No. 2. P. 78–80. 5. Potekaev N.N., Serov D.G., Dvoryankova E.V., Zhukovsky R.O. Effective therapy of mycoses of the feet with a single use of a new external form of terbinafine - a film-forming solution of Lamisil Uno // Clinical Dermatology and Venereology. 2008. No. 4. pp. 85–88. 6. Sergeev A.Yu., Ivanov O.L., Sergeev A.Yu. and others. Study of modern epidemiology of onychomycosis // Bulletin of Dermatology and Venereology. 2002. No. 3. P. 31–35. 7. Khaldin A.A., Tsykin A.A., Izyumova I.M. Clinical and etiological effectiveness of 1% Lamisil® spray in the treatment of fungal infections of large skin folds // Russian Journal of Skin and Venereal Diseases. 2007. No. 1. P. 56. 8. Baran R. Onychomycosis: the current approach to diagnosis and therapy. London: Malden MA, 1999. 9. Chen SCA, Sorrell TC New Drugs, Old Drugs. Antifungal agents // Med. J. Austral. 2007. Vol. 187. No. 7. R. 404–409. 10. Cribier BJ, Bakshi R. Terbinafin in the treatment of onychomycosis: a review of its efficacy in high-risk populations and in patients with nondermatophyte infections // BJ Dermatol. 2004. Vol.150. R. 414–420. 11. De Chauvin MF, Viguie-Vallanet C., Kienzler JL, Larnier C. Novel, single-dose, topical treatment of tinea pedis using terbinafine: results of a dose-finding clinical trial. Mycoses. 2008 Jan. Vol. 51(1). R. 1–6. 12. Deng S., Hu H., Abliz P., Wan Z., Wang A., Cheng W., Li R. A random comparative study of terbinafine versus griseofulvin in patients with tinea capitis in Western China. Mycopathologia. 2011 Nov. Vol. 172(5). R. 365–372. 13. Gianni C. Update on antifungal therapy with terbinafine // G Ital Dermatol Venereol. 2010 Jun. Vol. 145(3). R. 415–424. 14. Kikuchi I., Tanuma H., Morimoto K., Kawana S. Usefulness and pharmacokinetic study of oral terbinafine for hyperkeratotic-type tinea pedis // Mycoses. 2008 Nov. Vol. 51(6). R. 523–531. 15. Korting HC, Kiencke P., Nelles S., Rychlik R. Comparable efficacy and safety of various topical formulations of terbinafine in tinea pedis irrespective of the treatment regimen: results of a meta-analysis // Am J Clin Dermatol. 2007. Vol. 8 (6). R. 357–364. 16. Revankar SG, Nailor MD, Sobel JD Use of terbinafine in rare and refractory mycoses // Future Microbiol. 2008 Feb. Vol. 3(1). R. 9–17. 17. Schafer–Korting M., Schoellmann C., Korting HC Fungicidal activity plus reservoir effect allow short treatment courses with terbinafine in tinea pedis // Skin Pharmacol Physiol. 2008. Vol. 21(4). R. 203–210. 18. Schmid–Wendtner MH, Korting H. Topical terbinafine. Reduction of duration of therapy for tinea pedis // Hautarzt. 2008 Dec. Vol. 59 (12). R. 986–991. 19. Singal A., Khanna D. Onychomycosis: Diagnosis and management // Indian J Dermatol Venereol Leprol. 2011 Nov–Dec. Vol. 77(6). R. 659–672. 20. Stock I. Antimycotic therapy of Tinea pedis and other foot mycoses // Med Monatsschr Pharm. 2008 Jul. Vol. 31(7). R. 247–258. Van Duyn Graham L., Elewski BE. Recent updates in oral terbinafine: its use in onychomycosis and tinea capitis in the US // Mycoses. 2011 Nov. Vol. 54 (6). R. 679–685.
Overdose
If an overdose of Terbinafine in tablet form occurs, the patient may experience a rash , nausea , headache , vomiting , dizziness , epigastric pain, and frequent urination. It is important to perform gastric lavage; they also practice taking activated carbon and symptomatic treatment.
There is no data on an overdose of the drug in the form of external agents. If accidentally ingested as an ointment or cream, nausea, headache, dizziness, and epigastric pain may occur. In this case, symptomatic treatment is carried out; the use of activated carbon is indicated.
Composition of Terbinafine ointment
The main component is terbinafine hydrochloride. In one tube it is contained in the amount of 1g per 100g of composition. As additional substances, it contains rare cross-linked polyacrylic acid in the amount of 1.5 g, as well as 10 g of propylene glycol, 5 g of petroleum jelly, 0.4 g of sodium hydroxide. A large share of the volume is purified water - here it is 81g.
Terbinafine is available both as an ointment and as a gel or cream. The product for topical use is packaged in aluminum tubes, which are placed in cardboard packages, 1 piece each.
Interaction
When using Terbinafine, there may be an effect on the clearance of drugs whose metabolism involves the cytochrome P450 . These are Tolbutamide , Cyclosporine , oral contraceptives .
Increases the concentration of H2-histamine blockers in the blood plasma.
Slows down the excretion of Rifampicin , in turn, Rifampicin doubles its clearance.
Women who use oral contraceptives may experience menstrual irregularities.
Terfenadine inhibits the CYP2P6 isoenzyme, which leads to an obstruction of the metabolism of tricyclic antidepressants, beta-blockers, selective serotonin reuptake inhibitors, antiarrhythmic drugs, type B monoamine oxidase inhibitors, and antipsychotic drugs.
Under the influence of Terbinafine, the clearance of caffeine decreases by 21%, while its half-life increases by 31%.
Has no effect on the clearance of Digoxin , Phenazone , Warfarin .
When taken simultaneously with ethanol or with drugs that have a hepatotoxic effect, the likelihood of drug-induced liver damage increases.
special instructions
When treating onychomycosis for six weeks, it is not necessary to remove the affected nail plates.
It is important to consider that if the medication is not used regularly and its use is stopped prematurely, a relapse of the disease may occur.
The duration of treatment with Terbinafine may be affected by the presence of other diseases in the patient.
Systemic use of the drug in patients with onychomycosis is advisable only in cases of total damage to most nails, in the presence of subungual hyperkeratosis , and also in the absence of effect after local treatment.
People with liver disease may have reduced clearance of terbinafine.
During treatment, liver transaminase activity in the blood should be monitored.
Hepatitis and cholestasis may occur three months after starting treatment, but this occurs only in rare cases. If the patient develops signs indicating liver dysfunction, the medication is discontinued.
The drug should be prescribed with caution to patients suffering from psoriasis , as the medicine can provoke an exacerbation of this disease.
It is necessary to carefully observe all the rules of personal hygiene during the treatment process to prevent re-infection. After completion of treatment and during its process (two weeks after the start), you need to practice antifungal treatment of socks, stockings, and shoes.
Irritation can be caused by Terbinafine cream or ointment, which is why you should not allow these products to come into contact with your eyes. If the product gets into your eyes, rinse them immediately with clean water. If symptoms of irritation persist, you should consult your doctor.
In case of allergic manifestations, the medicine is discontinued.
Terbinafine in the treatment of onychomycosis in patients with somatic diseases
Onychomycosis of the feet and hands is one of the most common diseases. In the UK, 2.7% of the population suffers from onychomycosis: this figure accounts for 15–40% of all nail diseases and 10–30% of all dermatophytoses. 13% of men and 4.3% of women in Finland are diagnosed with onychomycosis [1, 2]. In Russia, 30–70% of people have onychomycosis pathogens isolated from the skin of their feet.
The source of onychomycosis pathogens is always a person who is sick or has recovered from this disease. And infection can occur in two ways: through direct contact with a patient (less often) or through the use of objects contaminated with pathogenic fungi (more often). Fungal cells are stable in the external environment: they can survive for a long time in socks and shoes, carpets, cracks in the floors of residential premises and public institutions (hotels, baths, showers, swimming pools, gyms), where sanitary and hygienic conditions are not observed. Pathogens of onychomycosis can persist even on particularly popular, heavily visited beaches. The high incidence of onychomycosis is explained not only by the widespread distribution of its pathogens and the contagiousness of the disease, but also by the low effectiveness of its treatment. Before the introduction of modern systemic antifungal drugs into medical practice, the possibility of obtaining a lasting therapeutic effect for onychomycosis did not exceed 40–50%.
Among the causative agents of foot mycoses, the most important are dermatophytes: Trichophyton rubrum (up to 80%) and Trichophyton mentagrophites. Onychomycosis, caused by dermatophytes, is a contagious disease. Its pathogens develop on keratinizing epithelium. In conditions of human immunodeficiency, they acquire parasitic properties and begin to feed not only on obsolete skin elements, but also on living tissues, causing an inflammatory reaction.
The threat of developing fungal infections of the feet increases with injuries, poor circulation in the lower extremities, and excessive sweating of the skin of the feet and hands. The state of the nervous and endocrine systems, type of metabolism, and body weight also matter. Women suffer from onychomycosis more often than men. Curvature of the fingers, deformation of the joints, abrasions, calluses lead to a change in the shape of the entire foot and a greater or lesser loss of its functions. The occurrence of an imbalance between the structure and function of the foot is accompanied by impaired blood circulation in it and a reduction in the supply of foot tissue with nutrients. This leads to a weakening of the protective functions of the skin of the foot.
The likelihood of nail disease increases with a combination of several risk factors, for example: increased function of the adrenal cortex, associated increase in body weight and sweat production, decreased immunity and contact with the source of the pathogen. Of the common diseases that most often make up the background of fungal nail diseases, the following should be indicated: Cushing’s disease or syndrome, diabetes mellitus, varicose veins of the lower extremities, thrombophlebitis, swelling in the legs of various origins, vitamin deficiencies. In recent years, one of the common causes of onychomycosis has become AIDS, which is generally characterized by fungal complications.
Treatment of onychomycosis
The use of local treatments for onychomycosis, even the most modern, makes it possible to obtain the desired result only with limited lesions of the nail plate. Cure of total nail damage is possible only by surgical removal of the nail plates or through the use of modern systemic agents. The choice of such products from a dermatologist and general practitioner is limited. The list includes only three names: itraconazole, fluconazole and terbinafine. Griseofulvin and ketoconazole, used in past years, are not currently used for the treatment of onychomycosis due to their low effectiveness and the risk of developing side effects and complications with long-term use. Only with the development of triazole derivatives and then allylamines was there a real breakthrough in the drug treatment of onychomycosis. The first such drug, itraconazole, was synthesized in 1980 [3].
In Russia, itraconazole in the form of orungal has been successfully used since 1996. And the first of the three listed drugs for systemic action in Russia was terbinafine, marketed under the name Lamisil [4].
The pharmacokinetics of terbinafine does not depend on the age, gender and other characteristics of the person. After oral administration, 40% of the drug is metabolized in the liver, then 90% binds to proteins and concentrates in tissues rich in proteins and keratin. The drug is easily absorbed in the small intestine, leaves the blood plasma in the next few hours and accumulates in keratin tissues, where it persists for weeks. Its fungistatic and fungicidal activity is realized by inhibiting squalene epoxidase, an enzyme system involved in the synthesis of ergosterol, which is responsible for the permeability of the fungal cell membrane. Impaired ergosterol synthesis leads to loss of cell cytoplasm. The cell loses its ability to reproduce and develop.
In 2000, a new terbinafine drug, exifin, was registered in Russia (Dr. Reddy,s Laboratories). When a new drug, an analogue of an existing one, appears, the question of its quality usually arises. There is an opinion that a new drug certainly has low therapeutic capabilities compared to the original drug and is capable of producing side effects that were not characteristic of the original drug.
In order to study the effectiveness of treatment and tolerability of exifin, we conducted an open, non-randomized clinical study of the results of its use in 54 patients with onychomycosis of the feet and hands caused by Tr. rubrum [8].
The condition for inclusion in the study group was the absence of treatment of patients with systemic antimycotic, hormonal, cytostatic or antibacterial drugs for a period of at least 4 weeks before the start of treatment with exifin. Our further studies were devoted to the peculiarities of the use and action of exifin in patients with underlying somatic pathology. A total of 27 such patients aged from 18 to 68 years received treatment with exifin, of which 15 were women, 12 were men.
The examination revealed the following background diseases: arterial hypertension - 5, diabetes mellitus type 1 - 6, diabetes mellitus type 2 - 8, bronchial asthma - 1, atherosclerosis of the aorta, coronary, cerebral vessels and great vessels of the extremities - 5, traumatic spondylitis with radicular syndrome and impaired motor function of the limbs - 1, Itsenko-Cushing syndrome - 1. Of particular interest were patients with diabetes mellitus, in whom carbohydrate metabolism disorders are accompanied by angiopathy of the peripheral vascular areas and polyneuropathy with impaired tissue trophism. Among patients with diabetes, every third person suffers from onychomycosis of the feet [9].
The duration of onychomycosis in the group ranged from 10 months to 30 years, the average duration was about 5 years. In all patients, the diagnosis of onychomycosis was verified by microscopic examination of scrapings from the nail plates in a 10% KOH solution and culture on Sabouraud's medium. Culture revealed the growth of the fungus Tr. in all patients. rubrum. Patients with other pathogens were not included in the study group.
The effectiveness of treatment was monitored once a month. Tolerability of the drug was monitored during the first 10 days of treatment. The treatment outcome was assessed after 3–4 months, and then after 8–9 months from the start of treatment.
Exifin was prescribed in tablets of 250 mg once a day. Treatment of onychomycosis of the feet was carried out for 80–112 days, depending on the severity of the disease. The given numbers are multiples of 16 - the number of tablets of the drug in one package. If necessary, exifin cream was additionally prescribed to the skin. The patient was advised to trim his nails as they grow and clean the affected part of the nail.
Recovery (good result) was confirmed by the restoration of the nail plates and the absence of the pathogen during microscopic examination of the material and growth in a nutrient medium. Incomplete restoration of the nail plates, detection of the pathogen by microscopy of scrapings in the absence of its growth on the nutrient medium made it possible to evaluate the result as satisfactory and was the basis for prolonging treatment. 3–4 months after the start of treatment with exifin, a clinical analysis of its results revealed a positive effect in most patients, but only after 8–9 months could the final result be assessed: during this time, natural regrowth (restoration) of the nail plates occurred in patients with a positive effect of treatment (25 patients out of 27). During mycological examination in the group of convalescents, the pathogen was not detected either microscopically or by cultural method.
The drug was well tolerated in all patients. Assessment of biochemical blood parameters during the course of therapy did not reveal negative dynamics. Thus, the present study, conducted on a limited number of patients, confirmed the high effectiveness of exifin in patients with onychomycosis of the feet caused by Tr. rubrum, against the background of somatic diseases with circulatory disorders in the extremities.
The research revealed the following conditions for the successful use of exifin in patients with diabetes mellitus and other underlying diseases:
- maximum compensation of impaired functions;
- study of the condition of the liver and kidneys (aminotransferases, bilirubin, creatinine) before and during treatment;
- readiness to extend the duration of treatment - up to 112 days or more;
- local therapy with exifin cream or other external antifungal drugs;
- means that increase microcirculation;
- vitamin therapy.
Based on the above, the following conclusions can be drawn.
- Exifin tablets in terms of effectiveness, harmlessness to the patient, and ease of use meet the requirements for modern drugs for the treatment of onychomycosis of the feet and hands.
- Exifin can be recommended by dermatologists and general practitioners for systemic treatment of onychomycosis caused by Tr. rubrum, in patients with underlying pathology accompanied by metabolic disorders and circulatory tissue disorders.
- In patients with diabetes mellitus and other underlying diseases, compared with patients without them, an increase in the duration of treatment for onychomycosis with exifin may be required.
- To optimize the results of treatment of onychomycosis with exifin, it is necessary to use vitamins and agents that improve the rheological properties of blood.
V. B. Antonov , Doctor of Medical Sciences, Professor of the Research Institute of Medical Mycology named after. P. N. Kashkina, SPbMAPO, St. Petersburg
Analogs
Level 4 ATX code matches:
Atifin
Binafin
Lamican
Griseofulvin
Analogues in tablets are the drugs Lamican , Onychon , Terbizil , Binafin , Exifin , Myconorm , Terbinafine hydrochloride , etc.
Analogues of Terbinafine for external use are the drugs Termicon , Lamisil Uno , Terbinox , Terbizil , Mikonorm , Lamitel , Terbinafine-MFF , etc.
Reviews of Terbinafine
Both reviews of Terbinafine tablets and reviews of cream and ointment appear online in most cases, positive. Patients write about the quick effect after starting to take Terbinafine and Terbinafine Teva tablets. With their help, it was possible to get rid of fungal diseases and restore the structure of the nails.
Those who have used Terbinafine ointment also leave positive reviews, noting that the product is inexpensive, but at the same time it allows you to completely cure fungal diseases and get rid of diaper rash in a few weeks. However, side effects develop very rarely.
Terbinafine cream - instructions
Unlike ointment, cream does not penetrate so deeply into the skin. Part of the applied layer is often rubbed off on clothing and surfaces with which the affected area of the body comes into contact. Therefore, the use of cream can be increased up to 3 times a day.
The advantage of the cream is its effectiveness even on moderately wet surfaces, which is especially important for fungal skin infections. If it is not possible to dry the surface due to the constant release of liquid, it is better to buy a cream, since the ointment in this case will be ineffective.
Terbinafine price, where to buy
The price of Terbinafine in tablets is from 210 rubles for 10 pieces. Terbinafine Teva tablets can be bought for an average of 130 rubles. (pack of 7 pcs.). The average price of Terbinafine cream is 80 rubles. per pack 15 g. The price of Terbinafine spray is from 300 rubles. for 30 ml.
Price of Terbinafine ointment in UAH. (Ukraine) – from 100 UAH. You can purchase the product in Dnepropetrovsk and other cities by order.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
ZdravCity
- Terbinafine Canon tablets 250 mg 10 pcs. JSC Canonpharma Production
RUB 149 order - Terbinafine cream 1% 15g VertexVertex AO
147 RUR order
- Terbinafine tablets 250 mg 14 pcs. JSC Canonpharma Production
RUB 271 order
- Terbinafine cream 1% 30gVertex AO
260 rub. order
- Terbinafine tablets 250 mg 14 pcs. JSC Medisorb
RUB 209 order
Pharmacy Dialogue
- Terbinafine-Teva (250 mg tablet No. 28) Teva
RUR 718 order
- Terbinafine-Canon tablets 250 mg No. 14Canonpharma Production
RUB 244 order
- Terbinafine-Teva (250 mg tablet No. 14) Teva
RUR 494 order
- Terbinafine-Canon (tab. 250 mg No. 14)Canonpharma Production
RUB 239 order
- Terbinafine (cream 30g)Vertex
RUB 234 order
show more
Pharmacy24
- Terbinafine-KV 250 mg No. 14 tablets PAT "Kiev Vitamin Plant", Kiev, Ukraine
80 UAH. order