1.General information
The term “acidosis” can be translated from Latin as “total acidification.” This is the name given to the decrease in the total hydrogen index (pH) in the body relative to its standard, natural values: 7.37 - 7.44. Maintaining the acid-base balance within these limits is one of the most important elements of homeostasis (constancy of internal conditions), since its strictly defined and fairly narrow range is necessary for the effective occurrence of all biochemical and neurotransmitter reactions, digestive processes, the functioning of symbiotic microflora, and many others.
Acidosis accompanies many diseases, pathological processes and conditions in which the circulation and removal of organic acids from the body is disrupted. The range of such conditions is very wide - from pregnancy and long-term malnutrition to severe infections and endocrine-metabolic disorders (for example, diabetes mellitus). Thus, acidosis is not an independent disease; This is a secondary syndrome that develops due to certain pathogenetic patterns.
A must read! Help with treatment and hospitalization!
Acidosis
In severe acidosis, when the content of bicarbonate ions in the plasma becomes very low, the urine pH drops below 5.5, the blood pH below 7.35, and the HCO3 concentration below 21 mEq/L. In the absence of pulmonary diseases, the partial pressure of carbon dioxide in arterial blood does not reach 40 mmHg. Art. With simple metabolic acidosis, it can decrease by about 1-1.3 mm Hg. Art. for every mEq/L decrease in plasma HCO3 levels. A greater drop in paCO2 indicates simultaneous primary respiratory alkalosis.
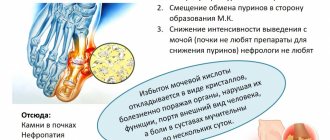
Many forms of metabolic acidosis are characterized by an increase in undetectable anions . The amount of serum undetectable anions (sometimes called anion gap or anion deficiency) is estimated by the difference between the serum sodium concentration and the sum of the chloride and bicarbonate concentrations. It is believed that normally this value ranges between 12 + 4 meq/l. However, it is derived from measuring electrolyte levels using a Technicon auto analyzer, which was widely used in the 1970s. Currently, most clinical laboratories use other methods that give slightly different figures. In particular, the normal serum chloride level is higher, and there are normally fewer undetectable anions - only 3-6 mEq/L. You should be aware of this and proceed from the limits of the standards established in the laboratory whose services are used in this particular case.
Metabolic acidosis may be associated with the accumulation of undetectable anions - for example, sulfate in renal failure, ketone bodies in diabetic or alcoholic ketoacidosis, lactate or exogenous toxic substances (ethylene glycol, salicylates). Metabolic acidosis with normal amounts of undetectable anions (hyperchloremic metabolic acidosis) is usually due to primary loss of bicarbonate through the gastrointestinal tract or kidneys (eg, renal tubular acidosis).
Diabetic acidosis is usually characterized by hyperglycemia and ketonemia. With hyperglycemia and non-ketone (according to routine clinical tests) acidosis, the content of lactic and/or p-hydroxybutyric acid in the blood is increased.
Ethylene glycol poisoning should be suspected in cases of unexplained acidosis if oxalate crystals are present in the urine.
Salicylate poisoning is characterized first by respiratory alkalosis and then by metabolic acidosis; the level of salicylates in the blood usually exceeds 30-40 mg%.
Since acidosis is often accompanied by hypovolemia, mild azotemia is often observed (blood urea nitrogen content 30-60 mg%). Greater increases in blood urea nitrogen, especially when combined with hypocalcemia and hyperphosphatemia, suggest renal failure as the cause of acidosis. Hypocalcemia is sometimes observed in septic shock. Changes in serum potassium levels during acidosis are discussed above (see disorders of potassium metabolism). In lactic acidosis, hyperkalemia is relatively rare unless there is concurrent renal failure and/or increased tissue breakdown.
2. Reasons
The main reasons for the shift in acid-base balance towards acidification include:
- smoking, drinking alcohol;
- malnutrition (regardless of reasons);
- insufficient fluid intake and dehydration (dehydration) of any other origin;
- intoxication with impaired digestive function of the gastrointestinal tract;
- carrying a pregnancy;
- metabolic disorders;
- cancer;
- hypoxia of any origin;
- a number of kidney diseases.
Visit our Nephrology page
Acid-base balance is an important parameter that is maintained in human blood within certain limits. This is necessary for the normal functioning of various body systems, the occurrence of biochemical reactions, and the optimal functioning of enzymes.
Acids are substances that can give off hydrogen ions, and bases (alkalis) are substances that attach these ions. The acidity and alkalinity of solutions is assessed on a pH scale from 0 (solutions of strong acids) to 14 (solutions of strong alkalis). On the pH scale, neutral acidity is 7.
Normal blood acidity is 7.35 – 7.45 on the pH scale. A shift of this indicator below 7.35 indicates acidosis (a shift in the acid-base balance of the blood towards increasing acidity). When the pH deviates above 7.45, alkalosis occurs (excess of substances with alkali properties in the blood).
During the process of metabolism in the body, products are formed in large quantities that can cause a change in this parameter. The main role in the regulation of acid-base balance belongs to the lungs, kidneys and blood buffer systems.
During breathing, carbon dioxide is released through the lungs, which is formed during the metabolic process in the body. Carbon dioxide, when combined with water, forms carbon dioxide, therefore, if there is an excess of it in the blood, acidosis develops, and if the concentration of carbon dioxide is insufficient, alkalosis occurs.
The kidneys remove excess acids and alkalis from the body with urine. At the same time, these organs, within certain limits, can regulate the amount of acids and bases released and absorbed back, due to which the pH level in the blood is regulated.
Blood buffer systems are solutions of weak acids and alkalis, which combine with excess amounts of acids or bases (depending on the presence of acidosis or alkalosis) to neutralize them, thereby equalizing the pH level.
The cause of acidosis and alkalosis in most cases is the severe course of the underlying disease, in which the resulting changes in blood pH exceed the capabilities of the mechanisms regulating this parameter.
Synonyms Russian
Disorders of the acid-base balance of the blood, disturbances of acid-base homeostasis.
English synonyms
Acid-Base Disorders, Acid-Base Homeostasis.
Symptoms
Manifestations of acidosis and alkalosis are often masked by manifestations of the underlying disease, which caused a change in the acid-base balance of the blood.
Acidosis may have the following symptoms:
- nausea, vomiting
- increased breathing rate
- headache
- disturbance of consciousness (up to coma)
- drop in blood pressure (in severe forms of acidosis)
- heart rhythm disturbances.
Manifestations of alkalosis may include:
- headache
- dizziness
- depression of consciousness (up to coma)
- cramps in various muscle groups
- heart rhythm disturbances
General information about the disease
The acid-base balance in the blood is a vital parameter, the normal values of which are 7.35 - 7.45 on the pH scale.
A pH deviation below 7.35 indicates acidosis. When the pH shifts above 7.45, alkalosis occurs.
Depending on the causes of development, acidosis and alkalosis are divided into metabolic (metabolic) and respiratory (breathing).
Respiratory acidosis develops as a result of the accumulation of large amounts of carbon dioxide in the blood, which combines with water to form carbon dioxide. This causes an increase in blood acidity. This condition can develop with breathing disorders that cause decreased pulmonary ventilation.
This may be the result of lung diseases (for example, bronchial asthma), damage to the nervous system (for example, with brain injuries), diseases, muscles and nerves that lead to loss of the ability to make effective breathing movements (for example, with amyotrophic lateral sclerosis).
The opposite condition is respiratory alkalosis, which occurs when the lungs remove excess carbon dioxide from the body. The mechanism for the development of this type of alkalosis is based on an increase in the rhythm and depth of breathing.
Such breathing disorders can occur in the presence of pathology from various organs and systems (for example, injuries, brain tumors, lung diseases, cardiovascular failure).
Metabolic acidosis can develop for the following reasons:
- increased production of acids in the body. An increase in acid production in the body can be observed in conditions accompanied by metabolic disorders. For example, in diabetes mellitus, the use of glucose by cells is impaired due to a lack of the hormone insulin.
At the same time, the body begins to produce energy not from glucose, but from fats - an alternative way to obtain energy. The breakdown of fats in the liver is accompanied by the formation of large quantities of ketone acids, which leads to acidosis.
- impaired renal function. The kidneys play an important role in regulating the acid-base balance in the blood. In case of kidney diseases that lead to disruption of their functions, the processes of acid secretion and absorption of substances with an alkaline reaction may be disrupted, which can cause acidosis.
- loss of large quantities of alkalis with digestive juices. This condition can be observed with severe diarrhea, surgical interventions on the intestines.
- poisoning with poisons and toxic substances. The breakdown of these substances in the body can occur with the formation of large amounts of acids, which can cause acidosis.
The main causes of metabolic alkalosis are the following:
- loss of large amounts of acidic gastric contents. It can be observed with profuse vomiting, aspiration of stomach contents using a special probe.
- use of diuretics
- increased excretion of hydrogen ions by the kidneys. Such processes can be observed with an excess of the adrenal hormone aldosterone. Aldosterone is involved in the regulation of water and electrolyte balance in the body. An increase in its level can occur both in diseases of the adrenal glands and in pathologies of other organs (for example, heart failure).
Thus, the development of acidosis or alkalosis is often associated with the occurrence of pathological processes in which the resulting changes in acid-base balance exceed the compensatory capabilities of the body. In this case, an important role in treatment is played by the normalization of the patient’s condition for the underlying disease that caused the deviation in blood pH.
Who is at risk?
The risk group for developing acid-base balance disorders in the blood includes:
- persons suffering from lung diseases (for example, bronchial asthma)
- persons with kidney disease with impaired kidney function
- persons suffering from diabetes mellitus
- persons with damage to the nervous system (for example, brain injuries, strokes)
- persons who have suffered large losses of gastrointestinal contents (for example, with excessive vomiting, frequent loose stools)
- persons taking certain medications (eg, diuretics, aspirin)
- persons who abuse alcohol.
Diagnostics
Laboratory research methods play an important role in diagnosis, which allows one to establish the blood pH level, its gas composition, parameters of water-electrolyte metabolism and other vital indicators, monitoring and correction of which are necessary for these conditions.
Laboratory research:
- Determination of blood pH, blood gas composition. Determination of these parameters can be carried out using special devices - gas analyzers. The material for the study is arterial blood.
- General blood analysis. This analysis allows you to evaluate the main characteristics of blood composition: the number of red blood cells, hemoglobin, leukocytes, platelets. This study is not specific for diagnosing acidosis or alkalosis, but is necessary to identify the causes of changes in blood pH.
- General urine analysis with microscopy. This analysis shows the basic physicochemical properties of urine, its pH level, and the presence of pathological and physiological metabolic products.
- Glucose in blood plasma. Glucose is the main source of energy in the human body. An increase in blood glucose levels is observed in diabetes mellitus. Metabolic disorders that occur with this disease can lead to the development of acidosis.
- Potassium, sodium, chlorine in serum. Potassium, sodium, and chlorine are the main electrolytes in the human body that perform many functions. Among them are participation in the transport of substances into the cell and the removal of metabolic products from it, maintaining water and acid-base balance in the body.
- Alanine aminotransferase (ALT). Alanine aminotransferase is an enzyme found in many cells of the body. Most of it is concentrated in the liver. When the liver is damaged, the level of this enzyme in the blood increases. Impaired liver function can lead to changes in the acid-base balance in the blood.
- Creatinine and urea in blood serum. Creatinine and urea are the end products of protein metabolism in the human body. They are excreted from the body by the kidneys. If kidney function is impaired, an increase in these indicators may be observed. Kidney damage can lead to changes in the acid-base balance in the body.
Depending on the specific clinical situation, other laboratory tests may be required to identify the causes of acidosis or alkalosis (for example, determining the level of ketone bodies in the blood and urine, the concentration of lactate in the blood, etc.).
Research:
- Radiography. Chest x-rays can detect pathological changes in the lungs (for example, pneumonia) that result in changes in the rhythm and depth of breathing.
- Ultrasound examination (ultrasound). The method is based on the properties of ultrasound. Using ultrasound, you can visualize internal organs, identify changes in their structure, the presence of space-occupying formations (for example, cysts, tumors), which may be necessary to determine the causes of disturbances in the acid-base balance in the blood.
- Computed tomography (CT). The method allows you to obtain layer-by-layer
highly informative images of internal organs. This is of great importance for identifying the disease that caused acidosis or alkalosis (for example, respiratory failure resulting from cerebral hemorrhage).
Treatment
Treatment of disorders of the acid-base balance in the blood is aimed at treating the underlying disease that led to the development of acidosis or alkalosis. To normalize the pH level, intravenous administration of solutions that neutralize acids (for acidosis) or alkalis (for alkalosis) can be carried out.
Treatment of respiratory acidosis is aimed at restoring the rhythm and depth of breathing with the possible transfer of the patient to artificial ventilation (breathing using a special apparatus in cases of ineffective lung function).
For respiratory alkalosis, inhalation of air mixtures containing carbon dioxide can be used.
Prevention
There is no specific prevention of changes in the acid-base balance in the blood. Patients suffering from diseases that can cause changes in blood pH (for example, diabetes) should strictly follow the recommendations of their doctor and undergo regular examinations and treatment.
Recommended tests
- Blood pH determination
- Determination of blood gas composition
- General blood analysis
- General urine analysis with microscopy
- Blood plasma glucose
- Potassium, sodium, chlorine in serum
- Alanine aminotransferase (ALT)
- Serum creatinine
- Urea in serum
3. Symptoms and diagnosis
Since acidosis occurs against the background and as a result of some pathology, and with its significant severity or long-term course, in most cases the clinical picture of the underlying disease (condition) dominates.
In mild forms, acidosis can manifest itself as weakness, fatigue, nausea, and vomiting. In more severe cases, the so-called Kussmaul breathing (deep, noisy), blood pressure rises, heart rhythm is disrupted. If the pathological “vicious circle” causing acidosis is not interrupted at this stage, then very serious depression of the central nervous system may occur (up to confusion and loss of consciousness) or hypovolemic shock may develop (a life-threatening condition caused by a sudden rapid reduction in the volume of circulating blood and, accordingly, deficiency of tissue oxygenation). A further shift in pH to the acidic side (to 7.1 - 7.2 and below) inevitably leads to coma and then death.
Since the symptoms described above are mainly nonspecific (even in aggregate), acidosis and the degree of its severity are established by laboratory tests of blood and urine (clinical analysis, analysis of gas composition, serum analysis of electrolytes, etc.). It is much more important to identify not the acidosis itself, but its causes, and this must be done as soon as possible, since with some diseases leading to acidosis the patient’s condition can worsen to critical very quickly.
About our clinic Chistye Prudy metro station Medintercom page!
Publications in the media
Metabolic acidosis is characterized by a decrease in blood pH and a decrease in plasma bicarbonate concentration due to loss of bicarbonate or accumulation of acids other than carbonic acid (eg, lactic acid).
Types and reasons
Metabolic acidosis with increased anion gap.
• Ketoacidosis. Increased formation of ketone bodies (keto acids). Ketoacidosis develops as a complication of diabetes, prolonged fasting, and alcohol abuse.
• Lactic acidosis. Decreased oxygen delivery to tissues leads to increased lactate formation with concomitant severe metabolic acidosis. A characteristic feature of many conditions associated with low tissue perfusion (for example, shock and sepsis).
• Kidney failure. The accumulation of organic and inorganic anions, associated with a decrease in glomerular filtration rate (GFR), leads to an increase in the anion gap in severe renal failure.
• Poisoning with salicylates, methanol, ethylene glycol can lead to the accumulation of organic acids (eg, lactic acid).
Metabolic acidosis with normal anion gap (hyperchloremic metabolic acidosis).
• Renal bicarbonate loss •• Proximal tubular acidosis is characterized by decreased proximal tubular reabsorption of bicarbonate, resulting in excessive urinary excretion of bicarbonate. Causes: cystinosis, SLE, multiple myeloma, heavy metal poisoning, Wilson's disease and nephrotic syndrome •• Distal tubular acidosis. Causes: heavy metal poisoning, amphotericin B use, SLE, obstructive uropathy, Sjogren's syndrome and other conditions accompanied by hyperglobulinemia •• Hyperkalemic renal tubular acidosis. Hyperkalemia, particularly when combined with hyporeninemic hypoaldosteronism, is characterized by decreased ammonia excretion, decreased bicarbonate formation, and an inability to neutralize nonvolatile acids using buffer systems •• Loss of organic anions. In diabetic ketoacidosis, the loss of ketones in the urine leads to a decrease in the content of metabolic precursors of bicarbonate, because. ketones are metabolized in the liver using hydrogen ions in various redox reactions of the tricarboxylic acid cycle •• Inhibition of carbonic anhydrase. The diuretics acetazolamide and mafenide (used topically to treat burns) inhibit carbonic anhydrase and reduce proximal tubular reabsorption of bicarbonate.
• Loss of bicarbonate through the gastrointestinal tract (diarrhea, pancreatic fistula, ureterosigmoidostomy).
• Application of mineral acids. Hyperchloremic metabolic acidosis develops with the administration of hydrochloric acid or any of its metabolic precursors, including ammonium chloride, arginine hydrochloride, calcium chloride (orally only).
The clinical picture of metabolic acidosis is usually related to the underlying disease. When the blood pH is less than 7.2, a decrease in cardiac output may occur. Acidosis is sometimes accompanied by resistance to the vasoconstrictor effect of catecholamines, leading to the development of arterial hypotension. As the respiratory rate increases in response to a decrease in serum pH, Kussmaul respiration appears.
Diagnosis • Blood plasma electrolytes. Decreased bicarbonate and variable chloride values depending on whether acidosis is accompanied by a normal or increased anion gap • Arterial blood gas analysis. Decrease in bicarbonate level with a compensatory decrease in paCO2. In pure metabolic acidosis, paCO2 should be equal to the bicarbonate concentration times 1.5 plus 6–10 mmHg. A deviation from this value suggests the presence of a complication in the form of respiratory dysfunction (a paCO2 value lower than predicted suggests primary respiratory alkalosis; a paCO2 value higher than expected indicates a dysfunction of the respiratory function of central origin, leading to inadequate CO2 retention).
Treatment. For metabolic acidosis with a blood pH below 7.2, intravenous administration of sodium bicarbonate (44–88 mEq in 5% dextrose solution or 0.45% sodium chloride solution) until a pH value of 7.2 is achieved ( plasma bicarbonate concentrations of 8–10 mEq/L), while eliminating the cause of acidosis • The required amount of bicarbonate can be calculated using the formula. The approximate amount of sodium bicarbonate required to increase the plasma bicarbonate concentration from 6 mEq/L to 10 mEq/L is: 4 mEq/L ´ 0.5 ´ body weight in kg . When using this method of calculation, repeated measurements of plasma bicarbonate and blood pH are necessary • Underestimation of bicarbonate requirements can occur with continued loss of bicarbonate (proximal tubular acidosis) or sufficiently rapid formation of organic acid with consumption of the injected bicarbonate in buffer reactions (lactic acidosis). With proximal tubular acidosis, the constant need for bicarbonate is 2–4 mg/kg/day • Complications of infusion of sodium bicarbonate solution: volume overload, especially in cardiac and renal pathology, hypernatremia, hypokalemia, alkalosis.
ICD-10 • E87.2 Acidosis • P74.0 Late metabolic acidosis in the newborn
Lactic acidosis is an emergency condition with impaired carbohydrate metabolism
31.08.2021
Lactic acidosis or lactic acidotic coma is an emergency condition that can develop both against the background of diabetes mellitus and other
diseases whose pathogenetic basis is an increase in the level of uric acid in the organs and tissues of the body. Lactic acid level
significantly increases in states of deep anorexia and shock, for example, with cardiogenic or infectious shock.
Predisposing factors for the formation of ketoacidosis include:
• increased physical stress; • massive blood due to injuries; • infectious damage to the body with subsequent development of sepsis; • long-term use of high doses of oral sugar-lowering agents.
Features of clinical manifestations in lactic acid coma:
Although this type of coma is a rare pathology, it has an extremely harmful effect on the human body.
Almost eighty percent of patients are fatal.
In clinic , dysfunctions of the cardiovascular system come to the fore; patients progressively develop
signs of acute circulatory failure, due to a decrease in the tone of arterial vessels, a drop in blood pressure occurs, up to collapse.
Heart contractions slow down, which leads to acute hypoxia of the body. Coma is characterized by rapid breathing, which, over time, significantly
slows down until it comes to a complete stop.
The final diagnosis of lactic acidosis is established only by a doctor based on laboratory tests, and in the plasmatic part of the blood
a high level of lactic acid is determined, while the level of general blood decreases. With this type of coma, the level
Peripheral blood remains at normal levels.
Therapeutic measures for lactic acidosis are based on the rapid restoration of electrolyte balance and acidity levels in peripheral blood ,
for this purpose, a 2.5% sodium bicarbonate solution is administered parenterally.
In order to correct signs of circulatory disorders, restore normal vascular tone and thereby increase the patient’s blood pressure
drugs that stimulate vascular tone are administered. Some patients are indicated for a short course of short-acting insulin. With pronounced manifestations
In case of general intoxication of the body, the patient may undergo surgery .
Preventive measures for lactic acidosis are based on timely treatment of diseases associated with carbohydrate imbalance. Patients should
Strictly control the dosage of medications to lower glucose levels and avoid uncontrolled self-treatment. Timely treatment
infectious diseases and prevention of other predisposing factors. As stated above, this disease is considered to be extremely severe.
however, the prognosis for recovery remains questionable.
Published in Articles without category Premium Clinic
Interpretation of results: norm, reasons for increased performance
Reference values: 0.5-2.2 mmol/liter. The decrease in indicators is not critical. High levels of this substance can indicate various diseases, including life-threatening conditions. For example, an increase in indicators is observed in acute heart failure, leukemia, circulatory disorders, and diabetes. Only a doctor can make an accurate diagnosis and prescribe treatment. Also, an increase in indicators can be caused by emotional or physical stress, alcohol intoxication. Even in the absence of symptoms, if abnormalities are detected, the patient's condition should be monitored by a doctor.
When is a lactate test necessary?
The test results enable the doctor to determine the reasons for the disruption in the supply of oxygen to the organs and systems of the body. A blood lactate test is used to identify lactic acidosis and hypoxia and assess their severity. The lactate level makes it possible to differentiate the types of metabolic acidosis. For newborns, analysis is prescribed in case of asphyxia. In sports medicine, the study is used to monitor the condition of athletes during intense training.
The test is prescribed in the following cases:
- suspected lack of oxygen (symptoms such as rapid breathing, sweating, pale skin, shortness of breath, sudden weakness);
- impaired clarity of consciousness, fainting, fever, severe headaches, symptoms of meningitis;
- suspicion of diabetes, sepsis, heart or kidney pathologies, shock.