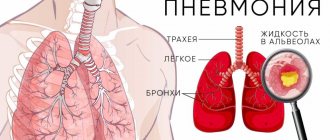
Bronchiectasis is an expansion of a separate section of the bronchi with changes in structure and function. Bronchiectasis is not an independent disease, but occurs as a result of many other diseases and conditions.
Bronchiectasis increases the patient's susceptibility to respiratory tract infections, which leads to frequent exacerbations and progression of the disease. Thus, bronchiectasis is a chronic progressive bronchopulmonary disease that requires constant medical supervision and supportive treatment, the volume of which increases with exacerbations.
Symptoms of bronchiectasis
Symptoms of bronchiectasis vary: from repeated episodes of respiratory tract infections, between which all symptoms of the disease completely disappear, to daily cough with sputum, the nature of which can vary from mucous (colorless) to mucopurulent (light yellow) and purulent (dark yellow, green or grey-green). Patients with bronchiectasis are characterized by a large volume of sputum - up to 100-200 ml per day, but sometimes the sputum is more scanty. From time to time, bloody streaks or clots may appear in the sputum, which is associated with trauma to the thinned bronchial wall during a hacking cough.
In rare cases, bronchiectasis is complicated by severe pulmonary hemorrhage requiring surgical intervention. When inflammation passes from the bronchial wall to the lung tissue, pneumonia can develop, and to the pleura - pain in the chest when breathing and coughing. In later stages of the disease, respiratory failure may develop.
Exacerbations of the inflammatory process in bronchiectasis are often accompanied by weakness, prolonged episodes of temperature (usually no higher than 37.2-37.50C), and weight loss.
Bronchiectasis. Complications
The altered walls of the bronchi, which have lost their elasticity and strength, and have lost their natural defense mechanisms, are suitable soil for the rooting and growth of pathogens (both those present in the body and those coming from outside). As a result of repeated inflammations and subsequent destruction of bronchial tissue, the situation worsens and can lead to:
- bleeding;
- recurring bronchitis and pneumonia;
- emphysema;
- secondary amyloidosis.
And ultimately - to the progression of heart and pulmonary failure.
Diagnosis of bronchiectasis
Bronchiectasis is detected by high-resolution computed tomography (CT) of the lungs. Conventional radiography, and especially fluorography, are not sensitive enough to diagnose this disease. According to CT scans of the lungs, it is sometimes possible to determine the cause - in congenital malformations of the lungs, tracheobronchomegaly, emphysema, tuberculosis, etc.
As a rule, to establish the cause, additional studies are required, the range of which can be quite wide, determined by the doctor during a conversation with the patient (collection of complaints and anamnesis), as well as based on the results of a CT scan of the lungs. Thus, if genetic diseases are suspected, a genetic analysis is carried out; if a fungal infection of the lungs is suspected, immunological studies (determination of antibodies to fungi) and special sputum cultures for fungal flora are performed.
What other studies are used in diagnosis?
Mandatory studies in patients with bronchiectasis are bacteriological analysis of sputum and a study of respiratory function (spirography, respiratory function or body plethysmography).
Bacteriological analysis of sputum (sputum culture)
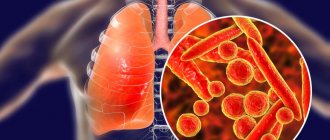
Bronchiectasis creates ideal conditions for the colonization of various microorganisms - the constant presence of bacteria on the surface of the bronchial mucosa in bronchiectasis. Long-term colonization of bacteria can cause inflammation even in the absence of other provoking factors (hypothermia, ARVI, etc.). This inflammation is manifested by frequent or persistent bronchitis with fever, weakness, sweating, and weight loss.
If the bacteria are not or cannot be removed from the bronchial tree, the inflammation becomes chronic with constant production of purulent sputum and lung damage. That is why it is important to regularly conduct bacteriological examination of sputum in order to control the composition and number of microorganisms present in the bronchi.
Pulmonary function test (PRF)
With chronic inflammation, the lumen of the bronchi narrows due to swelling of the bronchial mucosa, accumulation of mucus in the lumen of the bronchi, and sometimes bronchospasm (contraction of the muscles of the bronchial wall) can develop.
All these processes narrow the lumen of the bronchi and can cause shortness of breath. To diagnose these conditions, various methods of studying the function of external respiration are used. The simplest and most accessible method is spirometry, but more complex research methods are often required - measuring lung volumes (body plethysmography) and assessing the diffusion capacity of the lungs (the ability to pass oxygen from inhaled air into the blood). The results of these studies are important for prescribing treatment for a patient with bronchiectasis.
1.General information
Bronchiectasis, or bronchiectasis, is a serious, dangerous disease of the respiratory system. It consists of irreversible deformation (expansion) of the airways, the bronchi, most often in the lower parts of the bronchial tree, which is accompanied by a chronic purulent-inflammatory process and functional failure, expressed to one degree or another.
Bronchiectasis is by no means a sporadic rarity; it is detected with a frequency of about 1% in the general population (more often in children, up to 65% are boys). However, with regard to this disease, many etiopathogenetic, therapeutic, diagnostic and even classification issues remain unresolved today.
Thus, the debate is still ongoing about whether bronchiectasis should be considered an independent disease or a type of chronic pneumonia; whether this pathology is primary, or whether it is a secondary syndrome acquired as part of other diseases or under the influence of various causes. One way or another, the problem remains acute and relevant; it is being intensively studied by world pulmonology both in theoretical and in purely practical, therapeutic and diagnostic aspects.
A must read! Help with hospitalization and treatment!
Is it possible to cure the disease?
Bronchiectasis is a chronic progressive disease in which the quality of life of patients depends on the extent of lung damage, the degree of impairment of pulmonary function, the severity and frequency of exacerbations. There is no cure for this disease. But we are able to influence the rate at which the disease worsens. The rate of progression is largely determined by the nature of the chronic bronchial infection.
Thus, it is possible to slow down the progression of the disease and improve the patient’s quality of life with early diagnosis, identification and treatment of their cause, adequate treatment of chronic bronchial infections, prevention of exacerbations and regular medical supervision of the patient.
Are there surveillance programs for patients with bronchiectasis? Yes, they exist. Follow-up examinations with a doctor should be scheduled every 1 to 6 months, depending on the severity of the disease. Even if the patient’s condition is stable (in the remission phase), a general sputum analysis and bacteriological examination of sputum should be performed to assess the activity of inflammation in the bronchi. During the examination, the doctor should assess the severity of shortness of breath, the presence or absence of hemoptysis, general symptoms of inflammation (weakness, sweating, weight loss, temperature), listen to the lungs and, in case of severe impairment of pulmonary function, conduct a stress test (6-minute walk test).
Annual examination of a patient with bronchiectasis should include spirometry with a bronchodilator to assess the rate of decline in pulmonary function, a complete blood count with C-reactive protein (CRP) and immunoglobulin (Ig) A to assess the overall inflammatory response of the body.
At each visit to the doctor, it is advisable to measure saturation (oxygen saturation in the blood) using a pulse oximeter. If saturation decreases below 93%, it is recommended to perform a complete study of the gas composition of arterial blood to decide on the prescription of oxygen therapy.
For patients at high risk of disease progression, it is recommended to repeat CT scans of the lungs routinely once every 2 years. In addition, an X-ray of the lungs is performed annually, as well as if severe or life-threatening complications (pneumonia, pneumothorax) are suspected.
Bronchiectasis in children
Chronic lung diseases represent one of the most challenging problems in pediatrics. Many issues regarding this disease remain controversial to this day.
For half a century (starting with the works of S.P. Borisov, 1953), all chronic inflammatory lung diseases in children were essentially “absorbed” by the general concept of “chronic pneumonia”. Thus, bronchiectasis was considered as a stage in the formation of chronic pneumonia - from bronchitis to bronchiectasis [1]. In the International Statistical Classification of Diseases and Related Health Problems, 10th revision (WHO, 1995), bronchiectasis is presented under the heading (J. 47) in class X. In modern medical literature, the terms “bronchiectasis”, “bronchiolectasis”, “bronchiectasis” is often used interchangeably to refer to irreversible dilatation of the bronchi, accompanied by their anatomical defect. At the same time, the definition proposed by A. Ya. Tsygelnik (1968) remains relevant, according to which bronchiectasis is infected bronchiectasis [3].
According to the Ministry of Health, in Russia in 2001, 20 thousand 729 children and 5 thousand 629 adolescents with bronchiectasis were registered [4].
However, information about its prevalence among the population cannot be considered sufficiently accurate, since the most reliable sign of the disease—locally dilated bronchi—is diagnosed only using special research methods [5].
It should be emphasized that in recent decades there has been a decrease in the prevalence of bronchiectasis throughout the world. This is explained by a pronounced decrease in the number of childhood infections and cases of tuberculosis infection, as well as the expansion of diagnostic and treatment capabilities, successes in drug treatment of inflammatory lung diseases, and effective antibacterial therapy [6, 7].
Although bronchiectasis was first described by Laeneck almost 200 years ago, the mechanisms of its formation are still not fully understood. Among the most likely pathogenetic moments in the development of bronchiectasis, inflammation of the airways and impaired bronchial obstruction due to blockage or obstruction of the bronchial tube are of decisive importance. Each of these factors can become a trigger for the formation of bronchiectasis [8, 9]. In some cases, atelectasis of the lung tissue and fibrosis of the parenchyma appear to be important in the formation of bronchiectasis [3]. Usually, the appearance of bronchiectasis in children is associated with infectious diseases they have suffered (measles, whooping cough, respiratory infection, etc.). At one time, M.A. Skvortsov (1960) pointed out that bronchiectasis during acute infections in children can develop very quickly, within 1–2 days. Bronchiectasis also quickly forms in the presence of a foreign body in the bronchi [10].
In clinical practice, cylindrical, saccular and mixed bronchiectasis are distinguished. Cyst-shaped, spindle-shaped, and varicose bronchiectasis have also been described.
The initial signs of bronchiectasis usually appear in the first 3 years of a child’s life, and it is usually diagnosed in preschool children.
However, it should be noted that over the past 30 years there has been a tendency towards a milder course of the disease in children: the so-called “minor forms” predominate, which occur without symptoms of purulent intoxication or respiratory failure [7, 10, 11]. Purulent complications, previously considered characteristic of bronchiectasis and widely represented in the specialized literature (abscess formation of lung tissue, brain abscesses, amyloidosis) have become extremely rare [1, 3, 5].
The main clinical manifestations of bronchiectasis are repeated exacerbations of the inflammatory process in the lungs (up to 3-4 times a year); in children of the first years of life, a continuously relapsing course of the disease is often observed. The disease is characterized by a constant wet cough with sputum. Sputum is released mainly in the morning. Its quantity may be relatively small, and it may be separated in the form of separate spits. The separation of sputum “full of mouth”, as was previously observed in classical bronchiectasis, is rarely observed in children today.
During an exacerbation of the disease, patients may experience shortness of breath and oral crepitus. Hemoptysis, which was previously considered one of the main manifestations of the disease, is now more common in adult patients.
In patients with bronchiectasis, stable localized moist rales of various sizes are constantly heard. This is one of the most characteristic signs of bronchiectasis. Along with wet wheezing, patients may hear dry wheezing. In the presence of large bronchiectasis cavities, breathing over these zones may be amphoric in nature.
With bronchiectasis, various deformations of the chest are observed: most often, its flattening or retraction of one of its halves on the affected side. One of the characteristic clinical signs of bronchiectasis is considered to be thickening of the nail phalanges of the fingers (“drumsticks”), the so-called hypertrophic osteoarthropathy (Pierre Marie-Bamberger syndrome). However, in the modern course of the disease, this symptom occurs only in patients with widespread bronchiectasis and actively ongoing purulent endobronchitis.
A functional study of external respiration reveals obstructive and restrictive ventilation shifts. The degree of their severity depends on the prevalence of the pathological process.
Patients with a widespread process are characterized by a decrease in the value of forced expiration, the Tiffno index, a change in the structure of the lung volumes, an increase in the residual volume, and a decrease in the vital capacity of the lungs. In patients with a localized process, functional impairment may not be detected.
The use of radioisotope diagnostics in clinical pulmonology makes it possible to judge the state of regional lung functions and assess the nature of functional disorders in the area of the pathological focus and in other areas of the lung tissue.
X-ray and bronchological examination methods are of decisive importance in the diagnosis of bronchiectasis [12, 13]. Bronchiectasis, especially saccular and cystic, are detected on plain radiographs.
Clarification of the prevalence, extent of lesions and anatomical characteristics of bronchiectasis requires bronchography. It must be emphasized that even today bronchography has not lost its importance and continues to be the “gold standard” for diagnosing bronchiectasis [12, 13]. With the development of flexible fiber optics, selective bronchography has become possible [14].
It should be noted that the modern level of development of computer technology has led to the widespread use of high-resolution computed tomography (General Electric, Hitachi, Philips equipment) in the diagnosis of bronchiectasis. This method is widely used in children to diagnose bronchiectasis. High-resolution computed tomography can detect bronchiectasis that is not diagnosed even with bronchographic examination. The sign is considered reliable if the internal diameter of the peripheral bronchus is 2 times larger than the diameter of the previous pulmonary duct. Computed tomography allows one to measure the actual absolute dimensions of the bronchi [15].
Bronchiectasis lesions are most often localized in the lower lobes. The lower lobe of the left lung, lingular segments, and the middle lobe of the right lung are most often affected [12].
In bronchiectasis, the ciliary epithelium of the respiratory tract is lost and replaced by squamous or cubital epithelium. In cylindrical bronchiectasis, focal destruction of elastic tissue, edema and cellular infiltration of the surrounding parenchyma are determined; with more pronounced manifestations of the disease (saccular bronchiectasis), the damage concerns muscles and cartilage. Endarteritis develops in the pulmonary parenchyma and peribronchial tissue adjacent to bronchiectasis. Another characteristic sign is that near the distal subsegmental bronchi, anastomoses appear between the bronchial and pulmonary arteries [3, 5, 6].
Morphological lesions of the bronchi are accompanied by endobronchial changes. The nature and severity of endobronchitis depend on the period of the disease, the activity of the process, the prevalence of morphological changes, and the age of the sick child.
In almost all children suffering from bronchiectasis, bronchoscopy reveals catarrhal-purulent or purulent endobronchitis [16]. Inflammation of the bronchial mucosa is accompanied by a disruption of the structure and function of the ciliated epithelium and thereby causes a disruption of mucociliary transport. This, in turn, contributes to the persistent course of the inflammatory process in the respiratory tract.
It is fundamentally important to emphasize that bronchiectasis in children as a separate nosological form must be distinguished from bronchiectasis, which is a manifestation of other diseases.
The range of diseases and conditions in which bronchiectasis is detected is so wide that some authors consider the concept of “bronchiectasis” to be more pathological and morphological than clinical and nosological [17].
Thus, the basis for the formation of bronchiectasis can be congenital and hereditary diseases. According to the pulmonology clinic of the Moscow Research Institute of Pediatrics and Pediatric Surgery, congenital anomalies of the bronchopulmonary system are detected in 8–10% of patients with chronic inflammatory lung diseases [18].
Bronchiectasis can be a manifestation of congenital malformations of the structural elements of the walls of the trachea, bronchi and bronchioles, morphologically associated with the absence, deficiency or disorganization of cartilaginous or elastic tissues [19, 20, 21].
This group of defects includes Williams-Campbell syndrome, first described in 1960, in which, due to congenital generalized absence or underdevelopment of the cartilage of the segmental and subsegmental bronchi, “ballooning” bronchiectasis, bronchomalacia and bronchiolectatic emphysema occur. Bronchiectasis is also detected in tracheomegaly [20, 21].
Among all the causes of bronchiectasis, cystic fibrosis occupies a special place. It is believed that more than half of currently detected bronchiectasis is associated with a systemic genetically determined disease - cystic fibrosis [22]. This disease affects the exocrine glands of the bronchopulmonary system and intestinal tract. Obstruction of the bronchial lumen with viscous secretions creates conditions for the occurrence of a continuously recurrent inflammatory process in the lungs at an early age. Bronchopulmonary lesions in cystic fibrosis, as a rule, determine the clinical picture of the disease and its outcome.
Bronchiectasis is one of the main signs of primary ciliary dyskinesia, which is one of the hereditary lung diseases [23, 24]. The basis of this pathology is a defect in the structure of the cilia of the ciliated epithelium, which causes a violation of their function, and often complete immobility. This leads to disruption of the mucociliary cleansing function, and the subsequent layering of infection leads to an inflammatory process in the bronchi and nasopharynx. It is from these positions that the chronic inflammatory process in the respiratory tract and the formation of bronchiectasis are currently explained in the classic variant of primary ciliary insufficiency - Kartagener syndrome, which is characterized by a triad of symptoms: reverse arrangement of internal organs, bronchiectasis and sinusitis [25].
Some hereditary syndromes based on connective tissue pathology (Ehlers-Danlos syndrome, Marfan syndrome) may be accompanied by the presence of bronchiectasis [26, 27]. The question of the origin of bronchiectasis in a1-antitrypsin deficiency is being discussed: do they arise primarily or are they associated with the emphysema characteristic of this disease? [28]
Often bronchiectasis accompanies various forms of primary immunological deficiency. We are talking about immunodeficiencies with a predominant deficiency of antibodies: including autosomal recessive agammaglobulinemia (Swiss type), X-linked agammaglobulinemia (Bruton); selective IgA deficiency; combined immunodeficiencies; common variable immunodeficiency; other specific disorders, in particular those associated with a defect in the phagocytic system, are chronic granulomatosis in children [29].
In recent years, due to the increase in the number of patients with acquired immunodeficiency syndrome, the number of cases of bronchiectasis in these patients is increasing [30].
The formation of proximal bronchiectasis is pathognomonic for allergic bronchopulmonary aspergillosis, a disease based on sensitization to mold fungi of the genus Aspergillus that colonize the bronchial lumen [31]. Damage to the bronchi is caused by chronic allergic inflammation of the airways and the formation of specific dense mucus consisting of eosinophils, other allergic inflammatory cells and fungal hyphae [32].
All these diseases require special diagnostics, which determines the management and treatment of patients.
As for bronchiectasis, in recent years preference has been given to conservative treatment methods that are aimed at suppressing infection and restoring bronchial patency. Therapeutic tactics depend on the severity of the clinical manifestations of the disease, as well as the location and extent of the affected areas.
Antibacterial therapy is carried out in the acute phase of the disease, taking into account the bacteriological characteristics of sputum and the sensitivity of the identified microorganisms. Haemophilus influenzae, Streptococcus pneumoniae, and Moracella cataralis are most often found in the sputum of patients with bronchiectasis [33].
Considering the most likely range of microorganisms, in empirical antibiotic therapy for bronchiectasis, preference is given to modern penicillins with an extended spectrum of activity (amoxicillin, ampicillin), inhibitor-protected penicillins (amoxicillin/clavulanate, ampicillin/sulbactam), second and third generation cephalosporins (cefuroxime, cefaclor, cefotaxime, ceftriaxone , ceftazidime), as well as modern macrolides (azithromycin, josamycin, roxithromycin, spiramycin). In recent years, in addition to oral and parenteral antibiotics, administration of antibiotics through a nebulizer has also begun to be used [5, 6].
Bronchoscopic sanitation with the introduction of antibiotics through a bronchoscope (Pentax, Lomo, Karl Storz) has a significant clinical effect [16].
It should be noted that, in addition to modern antibiotic drugs, the pharmacotherapy of bronchiectasis uses drugs whose action is aimed at reducing bronchial hypersecretion, improving the drainage function of the bronchi and mucociliary clearance, as well as drugs with anti-inflammatory and bronchodilator effects.
Mucoactive drugs with secretolytic action include derivatives of the alkaloid vasicine (bromhexine, lazolvan), of which the most popular are bromhexine and its metabolites - lazolvan, ambroxol, ambrosan, chalixol. These drugs have a mucolytic effect associated with the depolymerization of mucoprotein and mucopolysaccharide fibers. It is important that the liquefaction of sputum is practically not accompanied by an increase in its volume. The drugs are used both orally and inhalation (via a nebulizer) [34].
Among the drugs that have a mucoregulatory effect, it is worth noting drugs based on carbocysteine - bronchobos, mucodin, mucopront, fluifort. They provide high mucociliary efficiency and are well tolerated; they practically do not irritate the gastric mucosa [34].
It is necessary to take into account that mucolytic drugs - cysteine derivatives with a free thiol group (acetylcysteine, fluimucil, exomyuk) - should be used only with significantly increased viscosity and elasticity of sputum. These drugs can make the secretion excessively liquid, resulting in a possible risk of developing bronchorrhea, which is most dangerous in young children due to the threat of aspiration [34].
Herbal preparations with a reflex expectorant effect are still widely used in the practice of complex therapy of bronchiectasis. Among them are bronchicum, sinupret, travisil, roots of ipecac, licorice, marshmallow, elecampane, thermopsis herb, and thyme. These drugs reduce the viscosity of sputum and improve the escalatory function of the ciliated epithelium. Their administration in combination with secretolytics and mucoregulators is very effective. It should be noted that drugs of this group should be used with caution in patients with hypersensitivity to pollen [34].
In recent years, in the treatment of chronic bronchopulmonary diseases, including bronchiectasis, fenspiride (erespal) has been used, a drug that has a whole range of pharmacological properties aimed at suppressing inflammation in the respiratory tract and mucus hypersecretion [34].
There is evidence that in the treatment of patients with bronchiectasis, anti-inflammatory drugs are used, including inhaled corticosteroids (flunisolide, beclomethasone, budesonide, fluticasone). Inhaled corticosteroids reduce the infiltration of T cells and IL-8-producing cells within the bronchial mucosa [35].
Activities aimed at cleansing the bronchi of secretions (massage, physiotherapeutic measures, postural drainage) are important elements of complex therapy. At the same time, in patients with bronchiectasis, activation of endobronchial secretion and the addition of superinfection are prevented.
In recent decades, indications for surgical treatment have undergone significant changes. This, according to M.R. Rokitsky et al. (1997), is associated both with the expansion of diagnostic and conservative therapeutic options, and with the pathomorphism of lung diseases that previously required active surgical treatment [7].
Indications for surgical treatment are currently considered [36]:
- limited unilateral bronchiectasis with persistent focal infection and lack of effect from conservative therapy;
- life-threatening conditions associated with bronchiectasis, in particular bleeding.
The tactics of surgical intervention have also changed. If at the peak of surgical activity in the 1960s and 70s, extensive resections were performed with the removal of 9–10 segments, up to pneumonectomy, then in recent decades, preference has been given to segmental and polysegmental resections [11].
It should be borne in mind that surgical removal of morphologically altered areas of the lung does not always mean a cure for the disease. E. V. Klimanskaya et al. (1998) note that in half of the operated children, exacerbations of the disease are recorded during long-term follow-up [11].
For a long time, bronchiectasis was considered primarily a childhood disease. However, many clinicians emphasize that bronchiectasis in adults in many cases is a consequence of this pathology in children. It must be emphasized that even with a favorable course of bronchiectasis and clear clinical improvement, morphological changes in the lungs do not undergo reverse development. Persistent morphological changes are the basis for the continuation of the inflammatory process in the bronchopulmonary system when patients reach adulthood [16, 37].
In conclusion, it should be noted that bronchiectasis is not only a medical, but also a social problem that requires close attention from specialists in various fields, as well as observation of patients by both pediatricians and pulmonologists.
For questions regarding literature, please contact the editor.
N. S. Lev , Candidate of Medical Sciences N. N. Rozinova , Doctor of Medical Sciences, Professor Moscow Research Institute of Pediatrics and Pediatric Surgery, Moscow
Pulmonary rehabilitation for bronchiectasis
Our pulmonology department has developed a “Pulmonary Rehabilitation Program for Patients with Bronchiectasis.”
A course of complex therapy can replace bronchoscopic sanitation in patients with bronchiectasis.
- sputum becomes easily coughed up, coughing occurs naturally, medications are poured into the smallest bronchi, including antimicrobial agents.
- the introduction of drugs into the bronchus and removal of sputum is not invasive and traumatic.
- Due to the positive effect of drainage techniques and special exercises included in the course, lymphatic drainage of the bronchi and their blood supply improve. As a result, the protective properties of the mucous membrane of damaged bronchi and surrounding lung tissue are enhanced.
- There are no risks inherent in bronchoscopy: the risk of bleeding, damage and allergic reaction to anesthesia.
4.Treatment
The therapeutic strategy for bronchiectasis is determined by its stage and clinical severity.
The most universal means are mucolytics and antibiotics, postural drainage, physiotherapeutic methods (special massage, breathing exercises, etc. are effective). In more severe cases, immunomodulators and immunostimulants, oxygen therapy, and sanitizing bronchoscopy are prescribed; As a last resort, life-saving measure, they resort to surgery to restore airway patency.
Treatment of bronchiectasis
Treatment of a patient with bronchiectasis is aimed at improving the condition, preventing exacerbations, and, consequently, progression of the disease.
If the cause of bronchiectasis is known, then treatment should be aimed at eliminating it.
The main components of success in treatment:
- adequate antibiotic therapy;
- powerful mucolytic (sputum thinning) therapy;
- bronchodilators;
- breathing exercises, breathing simulators;
- complete nutrition.
In some cases, mainly with a limited prevalence of bronchiectasis, surgical treatment (removal of the affected area of the lung) is possible in a thoracic department or surgical hospital. For this, a consultation with a thoracic surgeon will be necessary.
All patients with bronchiectasis should receive an influenza vaccine annually in the fall, and once every 5 years - the Pneumo-23 anti-pneumococcal vaccine. Of course, vaccination, like any method of treatment, has its contraindications, but modern vaccines reduce them to a minimum and make it possible to safely vaccinate the vast majority of patients, including patients with bronchial asthma and other allergic diseases.
Our specialists
Chikina Svetlana Yurievna
Candidate of Medical Sciences, pulmonologist of the highest category. Official doctor, expert at Russian congresses on pulmonology.
30 years of experience
Kuleshov Andrey Vladimirovich
Chief physician, candidate of medical sciences, pulmonologist, somnologist, member of the European Respiratory Society (ERS).
Experience 26 years
Meshcheryakova Natalya Nikolaevna
Candidate of Medical Sciences, pulmonologist of the highest category, associate professor of the Department of Pulmonology named after. N.I. Pirogov.
Experience 26 years
Nikitina Natalia Vladimirovna
Deputy chief physician, pulmonologist, allergist of the highest category. Full member of the European Academy of Allergy and Immunology.
Experience 15 years