Compound
| Pills | 1 table |
| active substance: | |
| metoclopramide hydrochloride monohydrate | 10.54 mg |
| (equivalent to 10 mg metoclopramide hydrochloride) | |
| excipients: potato starch - 36.75 mg; lactose monohydrate - 76.65 mg; gelatin - 2.16 mg; silicon dioxide - 2.6 mg; magnesium stearate - 1.3 mg |
| Solution for intravenous and intramuscular administration | 1 ml |
| active substance: | |
| metoclopramide hydrochloride monohydrate | 5.27 mg |
| (equivalent to 5 mg metoclopramide hydrochloride) | |
| excipients: sodium sulfite - 0.125 mg; disodium edetate - 0.4 mg; sodium chloride - 8 mg; water for injection - 991.705 mg |
Cerucal 10 mg/2 ml No. 10 solution d/in.amp
INSTRUCTIONS FOR MEDICAL USE OF THE MEDICINE CERUKAL TRADE NAME CERUKAL INTERNATIONAL NON-PROPENTED NAME METOCLOPLRAMIDE DOSAGE FORM SOLUTION FOR INJECTION 10 MG/2 ML COMPOSITION ONE AMPOOULE CONTAINS THE ACTIVE NEW SUBSTANCE - METOCLOPLRAMIDE HYDROCHLORIDE 10 MG (EQUIVALENT TO METOCLOPLRAMIDE HYDROCHLORIDE MONOHYDRATE 10.54 MG), EXCIENTISTS: SODIUM SULPHITE ANHYDROUS, DISODIUM ETHYLENEDIAMINETETRAACETATE, SODIUM CHLORIDE, WATER FOR INJECTION. DESCRIPTION TRANSPARENT, COLORLESS LIQUID. PHARMACOTHERAPEUTIC GROUP DRUGS FOR THE TREATMENT OF FUNCTIONAL DISORDERS OF THE GASTROINTESTINAL TRACT. GASTROINTESTINAL TRACT MOTOR STIMULATORS. METOCLOPRAMIDE. ATC CODE A03FA01 PHARMACOLOGICAL PROPERTIES PHARMACOKINETICS VOLUME OF DISTRIBUTION IS 2.2 -3.4 L/KG. METABOLIZED IN THE LIVER. THE HALF-PERIOD IS FROM 3 TO 5 HOURS, IN CHRONIC RENAL FAILURE - 14 HOURS. EXCRETED BY THE KIDNEYS DURING THE FIRST 24 HOURS IN UNMODIFIED FORM AND IN THE FORM OF METABOLITES (ABOUT 80% OF A SINGLE DOSE TAKEN). PHARMACODYNAMICS METOCLOPRAMIDE IS A CENTRAL ANTAGONIST OF DOPAMINE RECEPTORS; ALSO HAS PERIPHERAL CHOLINERGIC ACTIVITY. TWO MAIN EFFECTS ARE NOTED: ANTIEMETITIVE EFFECT AND THE EFFECT OF ACCELERATING EMPTYING OF THE STOMACH AND SMALL INTESTINE. THE ANTIEMETITIVE EFFECT IS DUE TO THE EFFECT ON THE CENTRAL RECEPTORS OF THE BRAIN STEM (CHEMORECEPTORS - THE ACTIVATING ZONE OF THE VOMITIC CENTER), PROBABLY BY INHIBITION OF DOPAMINERGIC NEURONS. INCREASED PERISTALTICS IS ALSO PARTIALLY CONTROLLED BY THE CNS CENTERS, BUT ALSO MAY BE PARTLY INVOLVED BY A PERIPHERAL ACTION MECHANISM, ALONG WITH ACTIVATION OF POSTGANGLIONARY CHOLINERGIC RECEPTORS AND POSSIBLY DOPAMINE INJECTION ERGIC RECEPTORS OF THE STOMACH AND SMALL INTESTINE. INDICATIONS FOR USE - VOMITING AND NAUSEA OF VARIOUS GENESIS; — ATONIA AND HYPOTENSION OF THE STOMACH AND INTESTINES (IN PARTICULAR POSTOPERATIVE) METHOD OF APPLICATION AND DOSES THE SOLUTION CAN BE ADMINISTRATED INTRAVENOUSLY OR INTRAMUSCULARLY. INTRAVENOUS DOSE SHOULD BE ADMINISTERED AS A SLOW BOLUS INJECTION (OVER 3 MINUTES). ADULTS: CERUKAL IS PRESCRIBED INTRAVENOUSLY OR INTRAMUSCULARLY 10 MG 1-3 TIMES A DAY. THE MAXIMUM SINGLE DOSE IS 10 MG, THE MAXIMUM DAILY DOSE IS 30 MG. CHILDREN AGED 2 TO 5 YEARS: THE MAXIMUM SINGLE DOSE IS 0.1 TO 0.15 MG/KG BODY WEIGHT. THE MAXIMUM DAILY DOSE IS 0.5 MG/KG BODY WEIGHT. CHILDREN AGED 5 TO 9 YEARS: 2.5 MG TO 5 MG DEPENDING ON WEIGHT. MAXIMUM DAILY DOSE IS NOT MORE THAN 15 MG. CHILDREN AGED 9 TO 18 YEARS: MAXIMUM SINGLE DOSE 5 MG, MAXIMUM DAILY DOSE 30 MG. CHILDREN AGED 15 TO 18 YEARS: WEIGHTING MORE THAN 60 KG: MAXIMUM SINGLE DOSE 10 MG, MAXIMUM DAILY DOSE 30 MG. CERUCAL IS PRESCRIBED FOR CHILDREN ONLY IN THE CASE OF A CONFIRMED DIAGNOSIS, ACCORDING TO STRICT LIFE INDICATIONS! DOSING TABLE: DOSING TABLE: AGE (YEARS) WEIGHT (KG) DOSE (MG) FREQUENCY 2 - 3 10 - 14 1 TO 3 TIMES A DAY 3 - 5 15 - 19 2 TO 3 TIMES A DAY 5 - 9 20 - 29 2.5 TO 3 TIMES A DAY 9 - 18 30 - 60 5 UP TO 3 TIMES A DAY 15 - 18 MORE THAN 60 10 UP TO 3 TIMES A DAY EXAMINATION OF THE UPPER GASTROINTESTINAL TRACT ADULTS: CERUKAL IS PRESCRIBED 10 MG, OVER 10 MIN BEFORE START THE EXAMINATION, INTRAVENOUSLY, SLOWLY (OVER 3 MINUTES). CHILDREN AGED FROM 2 TO 18 YEARS: CERUCAL IS PRESCRIBED AT 0.1 MG/KG OF BODY WEIGHT, 10 MINUTES BEFORE THE START OF THE EXAMINATION, INTRAVENOUSLY, SLOWLY (OVER 3 MINUTES). FOR NAUSEA AND VOMITING CAUSED BY CYTOSTATIC DRUGS, CERUKAL IS PRESCRIBED INTRAVENOUSLY BY DRIP: SCHEME 1 SHORT-TERM DROP INFUSION (OVER 15 MINUTES) AT A DOSE OF 2 MG/KG WEIGHT HALF AN HOUR BEFORE TREATMENT WITH A CYTOSTATIC AGENT AND ALSO AFTER 1 ½, 3 ½, 5 ½ , AND 8 ½ HOURS AFTER USING THE CYTOSTATIC. SCHEME 2 LONG-TERM DROP INFUSION (OVER 1 HOUR) AT A DOSE OF 1 OR 0.5 MG/KG OF WEIGHT 2 HOURS BEFORE USE OF A CYTOSTATIC AGENT, THEN AT A DOSE OF 0.5 OR 0.25 MG/KG OF WEIGHT 24 HOURS AFTER USE CYTOSTATIC AGENT. FOR INFUSIONS, IT IS RECOMMENDED TO DILUT THE INJECTION SOLUTION OF CERUCAL IN 50 ML OF ISOTONIC SODIUM CHLORIDE SOLUTION OR 50% GLUCOSE SOLUTION. IN PATIENTS WITH SEVERE LIVER FAILURE WITH ASCITES, DUE TO INCREASED HALF-LIFE, HALF THE DOSE IS APPLIED. PATIENTS WITH SEVERE LIVER FAILURE SHOULD BE MONITORED FOR SIDE EFFECTS. IF THEY OCCUR, STOP USING THE DRUG IMMEDIATELY. THE DURATION OF TREATMENT WITH CYTOSTATICS DEPENDS ON THE SEVERITY AND COURSE OF THE DISEASE AND IS DETERMINED BY THE DOCTOR. THE MAXIMUM RECOMMENDED DURATION OF TREATMENT IS 5 DAYS. PATIENTS WITH IMPAIRED RENAL FUNCTION REQUIRE DOSAGE REGIMEN ADJUSTMENT ACCORDING TO CREATININE CLEARANCE. CREATININE CLEARANCE DOSE OF METOCLOPRAMIDE FROM 11 TO 60 ML/MIN. 10 MG 1 TIME A DAY UP TO 10 ML/MIN. 5 MG 1 TIME A DAY SIDE EFFECTS IN GENERAL, THE FREQUENCY OF REACTIONS CORRELATES WITH THE DOSE AND DURATION OF TAKING METOCLOPLRAMIDE. THE FOLLOWING REACTIONS HAVE BEEN REPORTED, ALTHOUGH IN MOST CASES THE DATA DO NOT ALLOW FREQUENCY ESTIMATION: VERY COMMON (≥ 1/10) - Drowsiness COMMON (≥ 1/100, <1/10) - DIARRHEA - ASTHENIA - EXTRA APYRAMIDAL DISORDERS (ESPECIALLY IN CHILDREN AND YOUTH AND/OR WHEN THE RECOMMENDED DOSE IS EXCEEDED, EVEN AFTER A SINGLE DOSE OF THE DRUG), PARKINSONISM, AKATHISIA - DEPRESSION - HYPOTENSION, ESPECIALLY WITH INTRAVENOUS ADMINISTRATION RARE (≥ 1/1000, <1/100 ) - GALACTOREA - CONVASIONS ESPECIALLY IN PATIENTS WITH EPILEPSY - CONFUSION NOT COMMON (≥ 1/1000, < 1/100) - BRADYCARDIA, ESPECIALLY WITH INTRAVENOUS ADMINISTRATION - AMENorrhea, HYPERPROLACTINEMIA - HYPERSENSITIVITY - DYSTONIA, DYKINESIA - REDUCED CONSCIOUSNESS ANIA - HALLUCINATIONS RARE (≥ 1/10,000, < 1/1000) - GALACTorrhea - CONVIVULSES, ESPECIALLY IN PATIENTS WITH EPILEPSY - CONFUSION UNKNOWN - METHEMOGLOBINEMIA - CARDIAC STILL OCCURING SHORTLY AFTER INJECTION APPLICATION - ATRIOVENTRICULAR BLOCK, LOD QT INTERVAL INTERVAL - GYNECOMASTIA - ANAPHYLACTIC REACTIONS (INCLUDING ANAPHYLACTIC SHOCK) ESPECIALLY WITH INTRAVENOUS ADMINISTRATION - TARDY DYKINESIA, WHICH MAY BE PERMANENT, DURING OR AFTER LONG-TERM TREATMENT, ESPECIALLY IN ELDERLY PATIENTS, MALIGNANT NEUROLEPTIC SYNDROME - SHOCK, syncope AFTER INJECTIONS - ACUTE ARTERIAL HYPERTENSION IN PATIENTS WITH PHEOCHROMOCYTOMA - SKIN REACTIONS SUCH AS RASHE, ITCHING, ANGIONEUROTIC EDEMA AND HURTICS * ENDOCRINE DISORDERS DURING LONG-TERM TREATMENT ASSOCIATED WITH HYPERPROLACTINEMIA (AMENORRHEA, GALACTORHEA, GYNECOMASTIA). THE FOLLOWING REACTIONS, SOMETIMES ASSOCIATED, APPEAR MORE FREQUENTLY WHEN THE DRUG IS USED IN HIGH DOSES: - EXTRAPYRAMIDAL SYMPTOMS: ACUTE DYSTONIA AND DYKINESIA, PARKINSONIC SYNDROME, AKATHISIA, EVEN AFTER A SINGLE DOSE OF THE DRUG ESPECIALLY IN CHILDREN AND YOUNG ADULTS - Drowsiness, depression of consciousness, confusion , HALLUCINATIONS. CONTRAINDICATIONS - HYPERSENSITIVITY TO THE ACTIVE SUBSTANCE OR ANY OF THE AUXILIARY SUBSTANCES - GASTROINTESTINAL BLEEDING, GASTROINTESTINAL PERFORATION OR MECHANICAL INTESTINAL OBSTRUCTION WHICH PRESENTS A RISK TO THE GIBLE INTESTINAL MOTORICS. - CONFIRMED OR SUSPECTED PHEOCHROMOCYTOMA, DUE TO THE RISK OF SEVERE ATTACKS OF HTN. — TARDIVE DYSKINESIA CAUSED BY NEUROLEPTICS OR METOCLOPLRAMIDE, IN A HISTORY. — EPILEPSY (INCREASED FREQUENCY AND INTENSITY OF SEIZURES) — PARKINSON’S DISEASE — SIMULTANEOUS USE WITH LEVODOPA OR DOPAMINERGIC AGONISTS — ESTABLISHED METHEMOGLOBINEMIA WHEN USING METOCLOPLRAMIDE OR NAD-CYTOCHR-B5 DEFICIENCY -REDUCTASES IN ANAMNESIS. — USE IN CHILDREN UNDER 2 YEARS — I AND III TRIMESTER OF PREGNANCY AND LACTATION DRUG INTERACTIONS METOCLOPLRAMIDE IS NOT COMPATIBLE WITH INFUSION SOLUTIONS WITH ALKALINE; COMBINATION TO AVOID ALCOHOL INCREASES THE SEDATIVE EFFECT OF METOCLOPRAMIDE. COMBINATION THAT MUST BE CONSIDERED METOCLOPRAMIDE INCREASES THE ABSORPTION OF DIAZEPAM, TETRACYCLINE, AMPICILLIN, PARACETAMOL, ACETYLSALICYLIC ACID, LEVODOPA, ETHANOL; SLOWS DOWN THE ABSORPTION OF DIGOXIN AND CIMETIDIINE. ANTICHOLINERGIC DRUGS AND MORPHINE DERIVATIVES ANTICHOLINERGIC DRUGS AND MORPHINE DERIVATIVES MAY HAVE MUTUAL ANTAGONISM WITH METOCLOPLRAMIDE IN EFFECT ON GASTROINTESTINAL MOTOR. DEPRESSANTS INHIBITING THE ACTIVITY OF THE CENTRAL NERVOUS SYSTEM (MORPHINE DERIVATIVES, TRANQUILIZERS, SEDATIVE H1 HISTAMINE RECEPTOR BLOCKERS, SEDATIVE ANTIDEPRESSANTS, BARBITURATES, POTENTIATE THE EFFECT OF METOCLOPRAMIDE. ROLEPTICS WHEN Metoclopramide is used in combination with other neuroleptics, a cumulative effect and extrapyramidal disorders may occur. METOCLOPLRAMIDE WITH SEROTONergic DRUGS SUCH AS SSRIs MAY INCREASE THE RISK OF DEVELOPING SEROTONIN SYNDROME DIGOXIN METOCLOPRAMIDE MAY REDUCED THE BIOAVAILABILITY OF DIGOXIN CAREFUL MONITORING OF DIG CONCENTRATIONS IS REQUIRED OXINE IN PLASMA CYCLOSPORINE METOCLOPRAMIDE INCREASES THE BIOAVAILABILITY OF CYCLOSPORINE (CMAX 46% AND EFFECT 22%).CAREFUL MONITORING REQUIRED PLASMA CONCENTRATIONS OF CYCLOSPORINE MIVACURIUM AND SUXAMETHONIUM METOCLOPLRAMIDE INJECTIONS MAY PROLONG THE DURATION OF NEUROMUSCULAR BLOCK (BY INHIBITING PLASMA CHOLINESTERASE). STRONG CYP2D6 INHIBITORS EXPOSURE TO METOCLOPLRAMIDE IS INCREASED WHEN CO-USED WITH STRONG CYP2D6 INHIBITORS SUCH AS FLUOXETINE AND PAROXETINE. SPECIAL INSTRUCTIONS NEUROLOGICAL DISORDERS EXTRAPYRAMIDAL DISORDERS POSSIBLY OCCUR, ESPECIALLY IN CHILDREN AND YOUNG PEOPLE, AND/OR WHEN HIGH DOSES OF METOCLOPRAMIDE ARE USED. THESE REACTIONS USUALLY OCCUR AT THE BEGINNING OF TREATMENT AND MAY APPEAR AFTER A SINGLE ADMINISTRATION. YOU SHOULD STOP TAKING METOCLOPLRAMIDE IMMEDIATELY IF SYMPTOMS OF EXTRAPYRAMIDAL DISORDERS OCCUR. THESE SYMPTOMS ARE USUALLY COMPLETELY REVERSIBLE WHEN TREATMENT IS DISCONTINUED, BUT SYMPTOMATIC TREATMENT MAY BE REQUIRED (BENZODIAZEPINES IN CHILDREN AND/OR ANTIPARKINSONIC ANTICHOLINERGIC DRUGS IN ADULTS). LONG-TERM TREATMENT WITH METOCLOPLRAMIDE MAY RESULT IN TARDIVE DYKINESIA, POTENTIALLY IRREVERSIBLE, ESPECIALLY IN ELDERLY PEOPLE. TREATMENT SHOULD NOT EXCEED THREE MONTHS BECAUSE OF THE RISK OF TARDIVE DYKINESIA. TREATMENT SHOULD BE DISCONTINUED WHEN CLINICAL SIGNS OF TARDIVE DYKINESIA APPEAR. MALIGNANT NEUROLEPTIC SYNDROME IS POSSIBLE WHEN TAKING METOCLOPRAMIDE IN COMBINATION WITH NEUROLEPTICS, AS WELL AS WHEN METOCLOPRAMIDE MONOTHERAPY. YOU SHOULD IMMEDIATELY STOP TAKING THE MEDICINE IF SYMPTOMS OF NEUROLEPTIC MALIGNAL SYNDROME OCCUR AND INstitute APPROPRIATE TREATMENT. WHEN PRESCRIBING THE DRUG, SPECIAL ATTENTION SHOULD BE PAID TO PATIENTS WITH CONCOMIENTED NEUROLOGICAL DISEASES AND TO PATIENTS RECEIVING TREATMENT WITH OTHER MEDICINES AFFECTING THE CNS. METOCLOPLRAMIDE MAY INCREASE PARKINSON'S DISEASE SYMPTOMS. PATIENTS WITH RENAL AND LIVER IMPAIRMENT WHEN USING THE DRUG IN PATIENTS WITH RENAL IMPAIRMENT AND IN PATIENTS WITH SEVERE LIVER IMPAIRMENT, A DOSAGE REDUCTION IS RECOMMENDED. PREGNANCY AND LACTATION A LARGE AMOUNT OF DATA ON THE USE OF THE DRUG IN PREGNANT WOMEN (OVER 1000 CASES OF USE) INDICATES THE ABSENCE OF DEVELOPMENTAL DAMAGES AND TOXIC EFFECTS ON THE FETUS. METOCLOPLRAMIDE MAY BE USED DURING PREGNANCY ONLY FOR STRICT IMPORTANT INDICATIONS. CONSIDERING THE PHARMACOLOGICAL PROPERTIES OF THE DRUG (AS FOR NEUROLEPTICS), WHEN USING METOCLOPLRAMIDE IN THE LATE TERMS OF PREGNANCY, EXTRAPYRAMIDAL SYMPTOMS IN NEWBORNS CANNOT BE EXCLUDED. METOCLOPLRAMIDE SHOULD NOT BE USED DURING LATE PREGNANCY. INFANTS SHOULD BE MONITORED WHEN USING METOCLOPRAMIDE. METOCLOPLRAMIDE IS EXTRACTED IN BREAST MILK AND IS NOT RECOMMENDED FOR USE DURING BREASTFEEDING. PECULIARITIES OF THE INFLUENCE OF THE DRUG ON THE ABILITY TO DRIVE A VEHICLE OR POTENTIALLY HAZARDOUS MECHANISMS WHEN TAKING THE DRUG, POTENTIALLY HAZARDOUS ACTIVITIES REQUIRING INCREASED ATTENTION SHOULD BE AVOIDED, RAPID PSYCHOLOGY INDICAL AND MOTOR RESPONSE (DRIVING VEHICLES, ETC.). An overdose of symptoms: drowsiness, confusion, irritability, anxiety, cramps, extrapyramidal motor disorders, impaired function of the cardiovascular system with bradycardia and arterial hypo- or hypertension. TREATMENT: IN MILD FORMS OF POISONING, SYMPTOMS DISAPPEAR 24 HOURS AFTER DISCONTINUATION OF THE MEDICINE (DEPENDING ON THE SEVERITY OF THE SYMPTOMATICS, IT IS RECOMMENDED TO ESTABLISH MONITORING OF THE PATIENT’S VITAL FUNCTIONS). EXTRAPYRAMIDAL DISORDERS ARE MANAGED BY SLOW ADMINISTRATION OF BIPERIDENE (DOSE FOR ADULTS - 2.5 - 5 MG; MANUFACTURER'S RECOMMENDATIONS SHOULD BE FOLLOWED). DIAZEPAM CAN BE USED. RELEASE FORM AND PACKAGING 2 ML OF THE DRUG ARE PLACED IN AMPOULES MADE OF TRANSPARENT, COLORLESS GLASS. 10 AMPOULES ARE PLACED IN A CONTOURED CELL PACKAGING MADE OF POLYVINYL CHLORIDE FILM. 1 PACKAGE TOGETHER WITH INSTRUCTIONS FOR USE IN THE STATE AND RUSSIAN LANGUAGES IS PLACED IN A PACK OF CARDBOARD. STORAGE CONDITIONS: STORE IN A PLACE PROTECTED FROM LIGHT, AT A TEMPERATURE NOT EXCEEDING 30ºС. KEEP OUT OF THE REACH OF CHILDREN! STORAGE LIFE 5 YEARS USE THE PREPARED INFUSION SOLUTION IMMEDIATELY. DO NOT USE AFTER THE EXPIRATION DATE STATED ON THE PACKAGING. CONDITIONS FOR RELEASE FROM PHARMACIES BY PRESCRIPTION MANUFACTURER MERCKLE GMBH, BLAUBEUREN, GERMANY REGISTRATION AUTHORITY HOLDER TEVA PHARMACEUTICAL INDUSTRIES LTD., PETACH TIKVA, ISRAEL ADDRESS OF THE ORGANIZATION RECEIVING IN THE TERRITORY OF THE REPUBLIC BLIKI KAZAKHSTAN CLAIMS FROM CONSUMERS ON THE QUALITY OF PRODUCTS (GOODS): RATIOPHARM KAZAKHSTAN LLP, 050059 (A15E2R), ALMATY, PR. AL-FARABI 17/1, BC NURLY-TAU, 5B, 6TH FLOOR. TELEPHONE: (727)3251615 ADDRESS OF THE ORGANIZATION IN THE TERRITORY OF THE REPUBLIC OF KAZAKHSTAN RESPONSIBLE FOR POST-REGISTRATION SUPERVISION OF THE SAFETY OF THE MEDICINE: RATIOPHARM KAZAKHSTAN LLP, 050059 (A15E2R), AL MATS, PR. AL-FARABI 17/1, BC NURLY-TAU, 5B, 6TH FLOOR. PHONE: (727)3251642, MOBILE +7(701)9240368, E-MAIL
Directions for use and doses
Pills
Orally, approximately 30 minutes before meals, with water.
Adults: the recommended dose is 1 tablet. (10 mg metoclopramide) 3-4 times a day.
Adolescents over 14 years of age: the recommended dose is 1/2–1 tablet. 2–3 times a day.
The maximum single dose is 2 tablets. (20 mg); maximum daily dose - 6 tablets. (60 mg).
Solution for IM or IV administration
IM or slow IV.
Adults and adolescents over 14 years of age: 10 mg (1 amp.) 1–3 times a day.
Children from 2 to 14 years: therapeutic dose is 0.1 mg/kg, maximum daily dose is 0.5 mg/kg
| Body weight, kg | Therapeutic dose, mg/ml | Daily dose, mg/ml |
| 50 | 5/1 | 25 |
| 30 | 3/0,6 | 15 |
| 20 | 2/0,4 | 10 |
To prepare for upper gastrointestinal examination
Adults and adolescents over 14 years of age: 1–2 amp. (10-20 mg metoclopramide) slowly over 1-2 minutes IV 10 minutes before the start of the study.
Children from 2 to 14 years: at the rate of 0.1 mg/kg, slowly, over 1–2 minutes IV, 10 minutes before the start of the study.
Prevention and treatment of nausea and vomiting caused by the use of cytostatics
Scheme 1. Short-term drip infusion (over 15 minutes) at a dose of 2 mg/kg half an hour before the start of treatment with a cytostatic agent, and then 1.5, 3.5, 5.5 and 8.5 hours after the use of cytostatics.
Scheme 2. Long-term drip infusion at a dose of 1 or 0.5 mg/kg/h, starting 2 hours before the use of a cytostatic agent, then at a dose of 0.5 or 0.25 mg/kg/h over the next 24 hours after use cytostatic agent.
The drip infusion is carried out briefly for 15 minutes after preliminary dilution of the dose of the drug Cerucal® in 50 ml of infusion solution.
The injection solution of Cerucal® can be diluted with isotonic sodium chloride solution or 5% glucose solution.
The drug Cerucal® is used throughout the entire period of treatment with cytostatic agents.
Common to both dosage forms
In case of renal dysfunction, the dose is selected according to the severity of renal dysfunction (see table).
Table
| Creatinine clearance, ml/min | Metoclopramide dose |
| to 10 | 10 mg 1 time per day |
| from 11 to 60 | daily dose 15 mg, divided into 2 doses (10+5 mg) |
In patients with severe liver failure and ascites, the dose of the drug is halved due to an increase in T1/2.
The duration of metoclopramide therapy depends on the disease and is usually 4–6 weeks. Long-term therapy, no more than 6 months, is possible in exceptional cases.
Buy Cerucal solution intravenously and intramuscularly 5mg/ml 2ml No. 10 in pharmacies
Tradename
: Cerucal®
International nonproprietary name
: metoclopramide
Dosage form
: solution for intravenous and intramuscular administration
Compound
1 ml contains:
active ingredient metoclopramide hydrochloride monohydrate 5.270 mg (in terms of metoclopramide hydrochloride 5.000 mg);
excipients: sodium sulfite anhydrous 0.125 mg, disodium edetate 0.400 mg, sodium chloride 8.000 mg, water for injection up to 1.0 ml.
Description:
transparent colorless solution.
Pharmacotherapeutic group
Antiemetic - central dopamine receptor blocker.
pharmachologic effect
A specific blocker of dopamine receptors, weakens the sensitivity of the visceral nerves that transmit impulses from the pylorus (pylorus) and duodenum to the vomiting center. Through the hypothalamus and parasympathetic nervous system, it has a regulating and coordinating effect on the tone and motor activity of the upper gastrointestinal tract (including the tone of the lower digestive sphincter at rest). Increases the tone of the stomach and intestines, accelerates gastric emptying, reduces hyperacid stasis, prevents duodenopyloric and gastroesophageal reflux, stimulates intestinal motility.
Pharmacokinetics
The volume of distribution is 2.2-3.4 l/kg.
Metabolized in the liver. The half-life is from 3 to 5 hours, in chronic renal failure - 14 hours. It is excreted by the kidneys during the first 24 hours unchanged and in the form of metabolites (about 80% of a single dose). Easily penetrates the blood-brain barrier and is excreted in breast milk.
Indications for use
Adults
— Prevention of postoperative nausea and vomiting. — Symptomatic treatment of nausea and vomiting, including acute migraine. - Prevention of nausea and vomiting caused by radiation therapy and chemotherapy. — To enhance peristalsis during X-ray contrast studies of the gastrointestinal tract.
Children
— Second line of treatment for postoperative nausea and vomiting. — Second line of prevention of delayed nausea and vomiting caused by chemotherapy.
Contraindications
— Hypersensitivity to metoclopramide and the components of the drug; - gastrointestinal bleeding, mechanical intestinal obstruction or perforation of the wall of the stomach and intestines, conditions in which stimulation of gastrointestinal motility poses a risk; - confirmed or suspected pheochromocytoma due to the risk of developing severe arterial hypertension; - tardive dyskinesia, which developed after treatment with antipsychotics or metoclopramide in history; - epilepsy (increased frequency and severity of seizures); - Parkinson's disease; - simultaneous use with levodopa and dopamine receptor agonists; - methemoglobinemia due to taking metoclopramide or a history of nicotinamide adenine dinucleotide (NADH) cytochrome b5 deficiency; - prolactinoma or prolactin-dependent tumor; - children under 1 year of age; - period of breastfeeding.
Carefully
When used in elderly patients; in patients with cardiac conduction disorders (including prolongation of the QT interval), impaired water and electrolyte balance, bradycardia, taking other drugs that prolong the QT interval, arterial hypertension; in patients with concomitant neurological diseases; in patients taking drugs that affect the central nervous system, depression (history); with renal failure of moderate and severe severity (creatinine clearance 15-60 ml/min); with severe liver failure; during pregnancy.
Use during pregnancy and breastfeeding
Pregnancy
Numerous data obtained on use in pregnant women (more than 1000 described cases) indicate the absence of fetotoxicity and the ability to cause malformations in the fetus. Metoclopramide can be used during pregnancy (I-II trimesters) only if the potential benefit to the mother outweighs the potential risk to the fetus. Due to pharmacological characteristics (similar to other antipsychotics), when using metoclopramide at the end of pregnancy, the possibility of developing extrapyramidal symptoms in the newborn cannot be excluded. Metoclopramide should not be used at the end of pregnancy (during the third trimester). When using metoclopramide, the condition of the newborn should be monitored.
Breastfeeding period
Metoclopramide is excreted in small amounts into breast milk. The possibility of adverse reactions in a child cannot be ruled out. The use of metoclopramide during breastfeeding is not recommended. If it is necessary to use the drug during lactation, breastfeeding should be stopped.
Directions for use and doses
Intravenous (IV) and intramuscular (IM).
IV injections should be administered as a slow bolus (at least 3 minutes).
Adults
Prevention of postoperative nausea and vomiting
Recommended single dose 10 mg (1 ampoule).
Second line treatment for postoperative nausea and vomiting. Second line prevention of delayed chemotherapy-induced nausea and vomiting
The recommended single dose of 10 mg (1 ampoule) is administered up to three times a day.
To enhance peristalsis during X-ray contrast studies of the gastrointestinal tract. As a means of facilitating duodenal intubation (to speed up gastric emptying and move food through the small intestine)
It is recommended to administer a slow (at least 3 minutes) intravenous bolus of 10-20 mg (1-2 ampoules) 10 minutes before the start of the study.
The maximum recommended daily dose is 30 mg or 0.5 mg/kg.
The period of administration of the drug in the form of injections should be as short as possible, followed by a transition to an oral or rectal dosage form.
Children's age from 1 to 18 years
Second line prevention of delayed chemotherapy-induced nausea and vomiting, second line treatment of postoperative nausea and vomiting
It is recommended to administer a slow (at least 3 minutes) intravenous bolus of 0.1-0.15 mg/kg up to 3 times a day.
The maximum daily dose is 0.5 mg/kg/day.
Dosage regimen
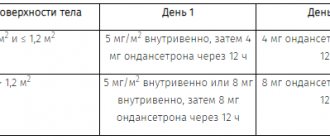
| Age (years) | Body weight (kg) | Dose (mg) | Frequency |
| 1-3 | 10-14 | 1 | Up to 3 times a day |
| 3-5 | 15-19 | 2 | Up to 3 times a day |
| 5-9 | 20-29 | 2,5 | Up to 3 times a day |
| 9-18 | 30-60 | 5 | Up to 3 times a day |
| 15-18 | more than 60 | 10 | Up to 3 times a day |
To enhance peristalsis during X-ray contrast studies of the gastrointestinal tract. As a means of facilitating duodenal intubation (to speed up gastric emptying and move food through the small intestine)
In children over 15 years of age
It is recommended to administer a slow (at least 3 minutes) intravenous bolus of 10-20 mg (1-2 ampoules) 10 minutes before the start of the study.
In children aged 1 to 15 years
It is recommended to administer a slow (at least 3 minutes) intravenous bolus at the rate of 0.1 mg/kg 10 minutes before the start of the study.
The maximum duration of treatment for the prevention of postoperative nausea and vomiting is 48 hours.
The maximum treatment period for preventing nausea and vomiting caused by chemotherapy is 5 days.
To avoid overdose, a minimum interval of 6 hours must be observed between doses, including in case of vomiting.
Elderly patients
In elderly patients, dose reduction may be required due to decreased renal and liver function.
Kidney failure
In patients with end-stage renal failure (creatinine clearance less than 15 ml/min), the daily dose should be reduced by 75%.
In patients with moderate or severe renal failure (creatinine clearance 15-60 ml/min), the dose should be reduced by 50%.
Liver dysfunction
In patients with severe hepatic impairment, the dose should be reduced by 50%.
Side effect
The frequency of adverse reactions is classified as follows: very common (≥ 1/10), common (≥ 1/100 - < 1/10), uncommon (≥ 1/1000 - < 1/100), rare (≥ 1/10000 - < 1/1000), very rare (< 1/10000), frequency unknown (cannot be estimated based on available data).
Disorders of the blood and lymphatic system: frequency unknown - methemoglobinemia, probably associated with a deficiency of the enzyme NADH-dependent cytochrome b5 reductase (especially in newborns), sulfhemoglobinemia (most often with the simultaneous use of high doses of sulfur-containing drugs, leukopenia, neutropenia, agranulocytosis).
Immune system disorders: uncommon - hypersensitivity; frequency unknown - anaphylactic reactions (including anaphylactic shock), allergic reactions (urticaria, maculopapular rash).
Endocrine system disorders*: uncommon - amenorrhea, hyperprolactinemia; rarely - galactorrhea; frequency unknown - gynecomastia.
*Endocrine disorders during long-term treatment are associated with hyperprolactinemia (amenorrhea, galactorrhea, gynecomastia).
Mental disorders: often - depression; infrequently - hallucinations; rarely - confusion.
Nervous system disorders: very often - drowsiness; often - asthenia, extrapyramidal disorders (especially in children and young patients and/or when recommended doses of the drug are exceeded, even after a single administration), parkinsonism, akathisia; uncommon - dystonia (including blurred vision and involuntary movement of the eye - oculogeric crisis), dyskinesia, impaired consciousness; rarely - seizures, especially in patients with epilepsy; frequency unknown - tardive dyskinesia, sometimes persistent, during or after long-term treatment, especially in elderly patients, neuroleptic malignant syndrome.
Cardiac disorders: infrequently - bradycardia; frequency unknown - cardiac arrest, which may be caused by bradycardia, atrioventricular block, sinus node block, prolongation of the QT interval on the electrocardiogram, ari.
Vascular disorders: often - low blood pressure; frequency unknown - cardiogenic shock, acute increase in blood pressure in patients with pheochromocytoma, transient increase in blood pressure.
Gastrointestinal disorders: often - nausea, diarrhea, constipation.
Renal and urinary tract disorders: frequency unknown - polyuria, urinary incontinence.
Genital and breast disorders: unknown frequency - sexual dysfunction, priapism.
Adverse reactions most common when using high doses of the drug
— Extrapyramidal symptoms: acute dystonia and dyskinesia, parkinsonism syndrome, akathisia developed even after using a single dose of the drug, especially in children and young patients (see section “Special instructions”). - Drowsiness, decreased level of consciousness, confusion, hallucinations.
Overdose
Symptoms
Extrapyramidal disorders, drowsiness, decreased level of consciousness, confusion, hallucinations, irritability, dizziness, bradycardia, changes in blood pressure, cardiac and respiratory arrest, abdominal pain.
Treatment
In the event of the development of extrapyramidal symptoms caused by overdose or for another reason, treatment is exclusively symptomatic (benzodiazepines in children and/or anticholinergic antiparkinsonian drugs in adults).
Symptomatic treatment and constant monitoring of cardiac and respiratory functions are required depending on the clinical condition of the patient.
There is no specific antidote.
Interaction with other drugs
The simultaneous use of metoclopramide with levodopa or dopamine receptor agonists is contraindicated due to the existing mutual antagonism.
Alcohol enhances the sedative effect of metoclopramide.
Combinations requiring caution
Due to the prokinetic effect of metoclopramide, the absorption of some drugs may be impaired.
M-anticholinergic agents and morphine derivatives have mutual antagonism with metoclopramide in terms of their effect on gastrointestinal motility.
Medicines that depress the central nervous system (morphine derivatives, tranquilizers, H1-histamine receptor blockers, antidepressants with sedative effects, barbiturates, clonidine and other drugs of these groups) can enhance the sedative effect under the influence of metoclopramide.
Metoclopramide enhances the effect of antipsychotics on extrapyramidal symptoms.
With concomitant oral use of metoclopramide and tetrabenazine, there is a possibility of dopamine deficiency, which may be accompanied by increased muscle stiffness or spasm, difficulty speaking or swallowing, anxiety, tremor, involuntary muscle movements, including facial muscles.
The use of metoclopramide with serotonergic drugs, such as selective serotonin reuptake inhibitors, increases the risk of developing serotonin syndrome (serotonin toxicity).
Metoclopramide reduces the bioavailability of digoxin. The concentration of digoxin in the blood plasma should be monitored.
Metoclopramide increases the bioavailability of cyclosporine (Cmax by 46% and exposure by 22%). It is necessary to carefully monitor the concentration of cyclosporine in the blood plasma. The clinical consequences of this interaction have not been established.
Metoclopramide exposure increases when used concomitantly with strong CYP2D6 inhibitors, such as fluoxetine and paroxetine. Although the clinical significance of this interaction has not been established, patients should be monitored for adverse reactions.
With the concomitant use of metoclopramide with atovaquone, the concentration of atovaquone in the blood plasma is significantly reduced (about 50%). Concomitant use of metoclopramide with atovaquone is not recommended.
With the concomitant use of metoclopramide with bromocriptine, the concentration of bromocriptine in the blood plasma increases.
Metoclopramide enhances the absorption of tetracycline from the small intestine.
Metoclopramide enhances the absorption of mexiletine and lithium.
Metoclopramide reduces the absorption of cimetidine.
special instructions
Caution should be exercised when using Cerucal® in elderly patients.
From the nervous system, extrapyramidal disorders may be observed, especially in children and young patients and/or when using high doses, usually developing at the beginning of treatment or after a single use. The use of Cerucal® should be stopped immediately if extrapyramidal symptoms occur. The reactions are completely reversible after cessation of treatment, but may require symptomatic therapy (benzodiazepines in children and/or anticholinergic antiparkinsonian drugs in adults).
To avoid an overdose of the drug Cerucal®, it is necessary to observe a minimum interval between doses of 6 hours, even in case of vomiting.
Long-term treatment with Cerucal® drugs may lead to the development of tardive dyskinesia, which is potentially irreversible, especially in elderly patients. The duration of treatment should not exceed 3 months due to the risk of developing tardive dyskinesia. If there are signs of tardive dyskinesia, treatment should be discontinued.
When metoclopramide was used simultaneously with neuroleptics, as well as with metoclopramide monotherapy, neuroleptic malignant syndrome was observed. It is necessary to immediately stop treatment with Cerucal® if symptoms of neuroleptic malignant syndrome appear and apply appropriate therapy.
Caution should be exercised when used in patients with concomitant neurological diseases and in patients taking drugs that affect the central nervous system.
When using the drug Cerucal®, symptoms of Parkinson's disease may also occur.
Cases of methemoglobinemia have been reported, which could be caused by a deficiency of the enzyme NADH-dependent cytochrome b5 reductase. In this case, taking the drug Cerucal® must be completely stopped immediately and appropriate measures taken.
Severe cardiovascular side effects have been reported, including vascular insufficiency, severe bradycardia, cardiac arrest, and QT prolongation.
Caution should be exercised when using the drug Cerucal® in elderly patients, patients with cardiac disorders (including prolongation of the QT interval), patients with fluid and electrolyte imbalance, bradycardia and in patients taking drugs that prolong the QT interval.
For moderate to severe renal failure and severe hepatic failure, a dose reduction is recommended (see section "Dosage and Administration").
Impact on the ability to drive vehicles and machinery
Care should be taken when driving vehicles and other machinery, because taking the drug may cause drowsiness and dyskinesia.
Release form
Solution for intravenous and intramuscular administration 5 mg/ml.
2 ml of the drug in a transparent glass ampoule (type I), with colored rings applied (top green and bottom blue) on the head of the ampoule and a white ring on the neck of the ampoule
or
in a transparent glass ampoule (type I), with colored rings applied (top green and bottom blue) on the head of the ampoule and a notch on the neck of the ampoule and a white dot above it
or in a transparent glass ampoule (type I), with colored rings applied (top green and bottom blue) on the head of the ampoule and a notch on the neck of the ampoule and a blue dot above it.
5 ampoules in an open blister pack.
2 blister packs with instructions for use are placed in a cardboard box, on which additional protective stickers can be applied
or
10 ampoules per open blister pack.
1 blister pack with instructions for use is placed in a cardboard box, on which protective stickers can additionally be applied.
Storage conditions
Store in a place protected from light at a temperature not exceeding 25°C.
Keep out of the reach of children!
Best before date
5 years. Do not use after the expiration date.
Vacation conditions
Dispensed by prescription.
Release form
Tablets, 10 mg. 50 tablets each in a brown glass bottle with a white LDPE stopper with the embossed inscription “AWD”; 1 fl. in a cardboard box.
Solution for intravenous and intramuscular administration, 5 mg/ml. 2 ml of the drug in transparent glass ampoules (type I), with colored rings applied (top - green, bottom - blue) on the head of the ampoule and a white ring on the neck of the ampoule or in transparent glass ampoules (type I), with colored rings applied rings (top - green, bottom - blue) on the head of the ampoule and a notch on the neck of the ampoule and a white dot above it. 5 ampoules in open blister packaging. 2 blister packs in a cardboard box.