Compound
1 tablet contains 1 mg benzoxonium chloride and 1 mg lidocaine hydrochloride .
Additionally: microcrystalline cellulose; sorbitol ; sodium saccharinate; macrogol 6000 ; corn starch; sodium chloride; lemon acid; orange flavor; Magnesium stearate.
100 ml spray includes: 0.2% benzoxonium chloride and 0.15% lidocaine hydrochloride .
Additionally: 0.0025% menthol, 10% ethanol (96%); 0.0581% hydrochloric acid (0.1 N); 15% glycerin ; 0.01% peppermint oil; up to 100% purified water.
Pharmacodynamics and pharmacokinetics
Theraflu LAR is a therapeutic agent from the group of antiseptics that also have local anesthetic activity, which are intended for use in ENT practice .
Quaternary ammonium salt - benzoxonium chloride the cationic structure it contains , is characterized by membranotropic efficiency and exhibits pronounced antibacterial (antimicrobial) activity towards mainly gram-positive microorganisms (it has a lesser effect on gram-negative strains of bacteria ). Benzoxonium also has antifungal and antiviral effects aimed at suppressing membrane viruses (including herpes , influenza and parainfluenza ).
The anesthetic lidocaine, when used topically for the treatment of ENT organs, reduces or completely eliminates painful sensations in the throat noted when swallowing.
The absorption of benzoxonium is practically zero. This drug is not detected in human plasma, and does not accumulate in tissues. Approximately 1% of the administered dose of benzoxonium is determined in the urine.
Lidocaine absorption when taken orally occurs in the oral cavity through its mucous membrane. Metabolic transformations are carried out in the liver during the “first pass”. The bioavailability of lidocaine with this method of administration is approximately 35%. The products of its metabolism are excreted in the urine; less than 10% of the drug is excreted in unchanged form.
TheraFlu LAR spray against viruses and sore throat, spray 30ml
A country
Italy
The country of production may vary depending on the batch of goods. Please check with the operator for detailed information when confirming your order.
Active substance
Benzoxonium chloride + Lidocaine
Description
TheraFlu LAR spray is a spray against viruses and sore throat, which has 5 actions at once*: • Antiseptic • Local anesthetic • Antibacterial • Antiviral • Antifungal For adults, during each procedure, 4 sprays (approximately 0.5 ml), multiplicity - 3 -6 times a day.
Children (starting from 4 years old) - no more than 2-3 sprays during each procedure, frequency - 3-6 times a day*. * Antiseptic, local anesthetic, antibacterial (Gram (+), Gram (-)), antifungal, antiviral. Instructions for medical use, RU LS-001853 dated 08/05/2011
Compound
(per 100 ml): Active ingredients: benzoxonium chloride 200 mg (0.2%), lidocaine hydrochloride 150 mg (0.15%). Excipients: ethanol 96% (v/v) 10 g (10%), glycerol 15000 mg (15%), hydrochloric acid 0.1 M 58.1 mg (0.0581%), peppermint leaf oil 10 mg (0.01%), levomenthol 2.5 mg (0.0025%), purified water up to 100 ml (100%)
Product description
Transparent colorless solution.
pharmachologic effect
Benzoxonium chloride is a quaternary ammonium salt (N - benzyl - N - dodecyl - N,N - di (2 - hydroxyethyl) ammonium chloride), due to its cationic structure, it has membranotropic activity and has a pronounced antibacterial effect against gram-positive and, to a lesser extent, gram-negative microorganisms. It also has antifungal and antiviral activity against membrane viruses (including influenza, parainfluenza and herpes viruses). Lidocaine is a local anesthetic that, during inflammatory processes, reduces pain in the throat when swallowing. Benzoxonium chloride is practically not absorbed. Approximately 1% of the administered dose is found in the urine; the concentration of the substance in the blood is practically undetectable. No accumulation of the substance was detected in body tissues. Lidocaine is absorbed orally and through the oral mucosa. Metabolized during the “first” passage through the liver; when administered orally, the bioavailability is approximately 35%. Metabolites are excreted in the urine, less than 10% of the substance is excreted unchanged.
Indications for use
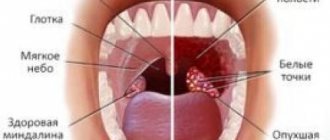
Infections of the oral cavity and pharynx, accompanied by sore throat: pharyngitis, laryngitis, catarrhal tonsillitis, stomatitis, ulcerative gingivitis. As an aid – chronic tonsillitis.
Contraindications
Hypersensitivity to the components included in the drug or ammonia compounds, children (up to 4 years), pregnancy, breastfeeding.
Use during pregnancy and lactation
The effect of the drug on reproductive function and fetal development was not detected in the experiment. Clinical experience with the drug in pregnant women is very limited, and therefore the drug should not be used during pregnancy. There is no clinical data on the penetration of the active component into breast milk. However, the drug is not recommended for use during breastfeeding.
Directions for use and doses
Locally. Holding the can vertically, spray it in the mouth. Adults: during each procedure, 4 sprays are carried out (approximately 0.5 ml), frequency 3-6 times a day. Children (starting from 4 years old) - no more than 2-3 sprays during each procedure, frequency - 3-6 times a day. The duration of treatment should not exceed 5 days. If there is no relief from symptoms of the disease within 5 days after starting to take the drug, you should consult a doctor.
Side effect
Classification of the incidence of adverse reactions: very often - more than 1/10 of prescriptions (≥ 10%); often - more than 1/100, but less than 1/10 prescriptions (≥ 1%, but ≤ 10%); infrequently - more than 1/1000, but less than 1/100 prescriptions (≥ 0.1%, but ≤ 1%); rarely - more than 1/10000, but less than 1/1000 prescriptions (≥ 0.01%, but ≤ 0.1%); very rarely - less than 1/10,000 prescriptions (≤ 0.01%). Local reactions: Uncommon: temporary irritation. Allergic reactions: Rarely: allergic reactions. Isolated cases of urticaria, swelling of the face and larynx have been reported. When using the drug for more than 2 weeks, a reversible brown discoloration of the tongue or teeth may occur.
Overdose
Accidental ingestion of large doses of the drug, like other ammonia compounds, can lead to nausea or vomiting. In case of poisoning, you should drink milk or eat egg whites beaten in water. The lidocaine content in TheraFlu LAR is insignificant and it cannot cause serious overdose symptoms.
Interaction with other drugs
The effectiveness of benzoxonium chloride is reduced when taking anionic agents such as toothpaste. Alcohol increases the absorption of benzoxonium chloride (drinking alcohol should be avoided during therapy).
special instructions
TheraFlu LAR spray is intended for topical use only.
Release form
Spray for topical use. 30 ml of the drug in a high-density polyethylene bottle with a spray device and a protective cap made of low-density polyethylene. The bottle, complete with a polypropylene spray nozzle and instructions for use, is placed in a cardboard pack. Secondary packaging is allowed to have a first-opening control.
Storage conditions
At a temperature not exceeding 30 C. Keep out of the reach of children.
Best before date
3 years. Do not use after expiration date
Indications for use
Theraflu LAR is indicated for the treatment of diseases of infectious and inflammatory etiology occurring in the pharynx and oral cavity, including:
- ulcerative gingivitis ;
- pharyngitis;
- catarrhal tonsillitis ;
- laryngitis;
- stomatitis;
- chronic tonsillitis (as a means of auxiliary therapy).
Side effects
Occasionally, when using Theraflu LAR, local reactions are observed, manifested by irritation , which are temporary.
In isolated cases, allergic manifestations , expressed by the occurrence of urticaria , as well as swelling of the larynx and/or face.
If the drug was used for more than 14 days, brown staining of the teeth and/or tongue
Instructions for use Theraflu LAR
For adult patients, the instructions for using Theraflu LAR tablets recommend dissolving 1 tablet of the drug every 2-3 hours. In case of severe symptoms of the disease, it is allowed to dissolve 1 tablet of Theraflu LAR every 60-120 minutes, but taking into account the maximum possible daily dosage of 10 tablets.
The daily dose of the drug for children (after 4 years) is a maximum of 6 tablets, maintaining the recommended dosage frequency - 1 tablet every 2-3 hours.
The tablets should be dissolved slowly until they are completely dissolved.
Theraflu LAR spray instructions for use recommend using patients over 4 years of age 3-6 times every 24 hours. Adult patients can perform up to 4 sprays once, children over 4 years old - no more than 2-3 sprays.
Theraflu LAR spray must be sprayed into the oral cavity while holding the canister with the drug strictly vertically.
If no relief of symptoms is observed within 5 days of using any of the dosage forms of Theraflu LAR, you should stop the treatment and consult your doctor.
Theraflu Lar
Registration number: LS-001853 Trade name: TeraFlu LAR Dosage form: spray for topical use International nonproprietary or generic name: Benzoxonium chloride + Lidocaine.
Composition (per 100 ml): Active ingredients: benzoxonium chloride 200 mg (0.2%), lidocaine hydrochloride 150 mg (0.15%). Excipients: ethanol 96% (v/v) 10 g (10%), glycerol 15000 mg (15%), hydrochloric acid 0.1 M 58.1 mg (0.0581%), peppermint leaf oil 10 mg (0.01%), levomenthol 2.5 mg (0.0025%), purified water up to 100 ml (100%).
Description: Transparent colorless solution.
Pharmacotherapeutic group: Antiseptic + local anesthetic.
ATX code: R02AA20.
Pharmacological properties Benzoxonium chloride is a quaternary ammonium salt (N - benzyl - N - dodecyl - N,N - di (2 - hydroxyethyl) ammonium chloride), due to its cationic structure it has membranotropic activity and has a pronounced antibacterial effect against gram-positive and, to a lesser extent , gram-negative microorganisms. It also has antifungal and antiviral activity against membrane viruses (including influenza, parainfluenza and herpes viruses). Lidocaine is a local anesthetic that, during inflammatory processes, reduces pain in the throat when swallowing.
Pharmacokinetics Benzoxonium chloride is practically not absorbed. Approximately 1% of the administered dose is found in the urine; the concentration of the substance in the blood is practically undetectable. No accumulation of the substance was detected in body tissues. Lidocaine is absorbed orally and through the oral mucosa. Metabolized during the “first” passage through the liver; when administered orally, the bioavailability is approximately 35%. Metabolites are excreted in the urine, less than 10% of the substance is excreted unchanged.
Indications for use Infections of the oral cavity and pharynx, accompanied by sore throat: pharyngitis, laryngitis, catarrhal tonsillitis, stomatitis, ulcerative gingivitis. As an aid – chronic tonsillitis.
Contraindications Hypersensitivity to the components included in the drug or ammonia compounds, children (up to 4 years), pregnancy, breastfeeding.
Use during pregnancy and breastfeeding No effect of the drug on reproductive function and fetal development was detected in the experiment. Clinical experience with the drug in pregnant women is very limited, and therefore the drug should not be used during pregnancy. There is no clinical data on the penetration of the active component into breast milk. However, the drug is not recommended for use during breastfeeding.
Method of administration and dosage Locally. Holding the can vertically, spray it in the mouth. Adults: during each procedure, 4 sprays are carried out (approximately 0.5 ml), frequency 3-6 times a day. Children (starting from 4 years old) - no more than 2-3 sprays during each procedure, frequency - 3-6 times a day. The duration of treatment should not exceed 5 days. If there is no relief from symptoms of the disease within 5 days after starting to take the drug, you should consult a doctor.
Side effects Classification of the frequency of occurrence of adverse reactions:
very often - more than 1/10 prescriptions (≥ 10%); often - more than 1/100, but less than 1/10 prescriptions (≥ 1%, but ≤ 10%); infrequently - more than 1/1000, but less than 1/100 prescriptions (≥ 0.1%, but ≤ 1%); rarely - more than 1/10000, but less than 1/1000 prescriptions (≥ 0.01%, but ≤ 0.1%); very rarely - less than 1/10,000 prescriptions (≤ 0.01%).
Local reactions: Uncommon: temporary irritation.
Allergic reactions: Rarely: allergic reactions. Isolated cases of urticaria, swelling of the face and larynx have been reported. When using the drug for more than 2 weeks, a reversible brown coloration of the tongue or teeth may occur.
Overdose Accidental intake of large doses of the drug, like other ammonia compounds, can lead to nausea or vomiting. In case of poisoning, you should drink milk or eat egg whites beaten in water. The lidocaine content in TheraFlu LAR is insignificant and it cannot cause serious overdose symptoms.
Interaction with other drugs The effectiveness of benzoxonium chloride is reduced when taking anionic agents, such as toothpaste, at the same time. Alcohol increases the absorption of benzoxonium chloride (drinking alcohol should be avoided during therapy).
Special instructions TheraFlu LAR spray is intended for topical use only.
Effect on the ability to drive vehicles and operate machinery . No effect.
Release form: Spray for local use. 30 ml of the drug in a high-density polyethylene bottle with a spray device and a protective cap made of low-density polyethylene. The bottle, complete with a polypropylene spray nozzle and instructions for use, is placed in a cardboard pack. Secondary packaging is allowed to have a first-opening control.
Storage conditions At a temperature not exceeding 30°C. Keep out of the reach of children.
Shelf life: 3 years. Do not use after expiration date.
Conditions of release Dispensed without a prescription.
The legal entity in whose name the registration certificate was issued and the organization accepting claims on the territory of the Russian Federation: GlaxoSmithKline Healthcare JSC. 123112, Moscow, Presnenskaya embankment, 10. Tel.; Fax
Toll-free hotline number8 800 333 46 94
Email:
The trademark is owned or used by the GlaxoSmithKline Group of Companies.
Manufacturer GSK Consumer Healthcare S.A., Switzerland/GSK Consumer Healthcare SA, Route de l'Etraz, 1260 Nyon, Switzerland. or Doppel Farmaceutici S.R.L., Italy/Doppel Farmaceutici SRL, Via Martiri Delle Foibe, 1 - 29016 Cortemaggiore (PC), Italy.
30104510.001
Overdose
In case of accidental or intentional oral administration of excessive dosages of Theraflu LAR (any of its dosage forms), nausea / vomiting . The mass fraction of lidocaine in the drug is insufficient to cause any negative symptoms.
As a treatment for the above conditions, it is recommended to eat egg whites , previously beaten in water, or drink milk .
Theraflu Lar spray for topical use (2mg+1.5mg)/1ml 30ml No.1
Name
Theraflu Lar spray local approx. (2mg+1.5mg) 1ml in vial. 30ml per pack. No. 1
Description
Transparent colorless solution with mint odor.
Main active ingredient
Benzoxonium chloride + lidocaine
Release form
30 ml of the drug in a high-density polyethylene bottle with a spray device and a protective cap made of low-density polyethylene. The bottle, complete with a polypropylene spray nozzle and instructions for use, is placed in a cardboard pack.
Dosage
2mg+1.5mg
pharmachologic effect
Benzoxonium chloride is a quaternary ammonium salt (N-benzyl N-dodecyl N, N-di (2-hydroxyethyl) ammonium chloride), due to its cationic structure, it has membrane-tropic activity and has a pronounced antibacterial effect against gram-positive and, to a lesser extent, gram-negative microorganisms. Benzoxonium also has antifungal and antiviral activity against membrane viruses (including influenza, parainfluenza and herpes viruses). Lidocaine is a local anesthetic that, during inflammatory processes, reduces pain in the throat when swallowing.
Pharmacokinetics
Benzoxonium chloride is practically not absorbed. In humans, approximately 1% of the administered dose is found in the urine; the concentration of the substance in the blood is not detected. No accumulation of the substance was detected in body tissues. Lidocaine is absorbed orally and through the oral mucosa. Metabolized during the “first” passage through the liver; when administered orally, its bioavailability is approximately 35%. Metabolites are excreted in the urine, less than 10% of the substance is excreted unchanged.
Indications for use
Infections of the oral cavity and pharynx: sore throat during colds, pharyngitis, laryngitis, stomatitis, ulcerative gingivitis. As an adjuvant for tonsillitis, including acute (tonsillitis) and chronic.
Directions for use and doses
Locally. Holding the can vertically, spray it in the mouth. Adults: during each procedure, 4 sprays are carried out (approximately 0.5 ml), frequency 3-6 times a day. Children (starting from 4 years old) - no more than 2-3 sprays during each procedure, frequency - 3-6 times a day. The duration of treatment should not exceed 5 days. If there is no relief from symptoms of the disease within 5 days after starting to take the drug, you should consult a doctor.
Use during pregnancy and lactation
The drug is not recommended for use during breastfeeding.
Interaction with other drugs
The effectiveness of benzoxonium chloride is reduced when taking anionic agents such as toothpaste. Alcohol increases the absorption of benzoxonium chloride (drinking alcohol should be avoided during therapy).
Contraindications
Hypersensitivity to caine or ammonia compounds. The use of the drug is not recommended for children under 4 years of age, in the first trimester of pregnancy and during breastfeeding. Use of the drug during pregnancy and breastfeeding. The effect of the drug on reproductive function and fetal development was not detected in the experiment. There have been no controlled studies in pregnant women, and therefore TheraFlu LAP should not be used during pregnancy, especially in the first trimester. There is no clinical data on the penetration of the active component into breast milk. However, the drug is not recommended for use during breastfeeding.
Compound
1 ml of spray contains: Active ingredients: Benzoxonium chloride 2 mg, lidocaine hydrochloride 1.5 mg. Excipients: Ethanol, peppermint oil, menthol, glycerin, hydrochloric acid, purified water.
Overdose
Accidental ingestion of large doses of TheraFlu LAP, like other ammonia compounds, may cause nausea or vomiting. In case of poisoning, you should drink milk or eat egg whites beaten in water. The lidocaine content of TheraFlu JIAP is negligible and is not likely to cause serious overdose symptoms.
Side effect
Sometimes when using TheraFlu LAP, local irritation is observed, which is temporary. Allergic reactions are rare. When using the drug for more than 2 weeks, a reversible brown coloration of the tongue or teeth may occur.
Storage conditions
Store at a temperature not exceeding 30 °C, out of the reach of children.
Analogs
Level 4 ATX code matches:
Falimint
Suprima-ENT
Ingalipt-N
Strepsils Plus
Strepsils
Ingalipt
Hexoral Tabs
Lisak
Efizol
Angilex
Kameton
Anzibel
Agisept
Yox
Lugol's solution with glycerin
Lugol
Laripront
Stopangin 2A
Stopangin
Septolete Neo
- Anti-angin;
- Hexalize;
- Agisept;
- Voqara;
- Gorpils;
- Hexasprey;
- Septolete;
- Ingalipt;
- Neo-Angin;
- Kameton;
- Rinza Lorsept;
- Tonsipret;
- Faringosept;
- Strepsils;
- Falimint.
Reviews
Patients who use TheraFlu LAR tablets or spray for the treatment of various painful conditions of the pharynx and oral cavity that have an infectious-inflammatory etiology generally characterize this drug on the positive side. In most cases, the drug copes well with pain in these organs and prevents the further development of bacterial infection . Naturally, treatment with one antiseptic may often be insufficient and require the addition of other medications from various pharmaceutical groups to the therapy.
Pharmacological properties of the drug Theraflu lar
benzoxonium chloride has a pronounced bacteriostatic and bactericidal effect against gram-positive and, to a lesser extent, gram-negative microorganisms. It is especially effective against pathogens of the oral cavity and throat, as well as microorganisms involved in the formation of dental plaque. Benzoxonium chloride also has antifungal and weak antiviral activity against membrane viruses (including influenza, parainfluenza and herpes viruses). Due to the cationic structure, benzoxonium chloride has membranotropic activity and a high degree of surface activity, which facilitates its penetration into the cells of infectious agents. Lidocaine is a local anesthetic that relieves pain during inflammatory processes in the throat and eliminates pain when swallowing. Does not irritate the mucous membrane, does not cause caries. Benzoxonium chloride is practically not absorbed. The degree of excretion in urine is low (approximately 1% of the applied dose within 24 hours). In blood plasma, the concentration of the substance is almost undetectable. No accumulation of the substance in body tissues was detected. Lidocaine is absorbed after oral administration in the oral cavity by the membranes of mucosal cells. Metabolized on the first pass through the liver. Bioavailability is about 35% when administered orally. Metabolism in the liver is rapid, metabolites are excreted in the urine.
Price, where to buy
The price of Theraflu Lar tablets No. 16 averages 200 rubles.
The average price of a 30 ml spray is 250 rubles.
- Online pharmacies in RussiaRussia
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- TheraFlu LAR spray for local use.
approx. fl. 30ml (with dispenser) Doppel Pharmaceutical S.r.L. RUB 325 order - TheraFlu LAR against viruses and sore throat with menthol flavor lozenges 20 pcsNovartis Urunleri
RUB 257 order
Pharmacy Dialogue
- TheraFlu LAR spray against viruses and sore throat, spray 30mlNovartis
RUB 311 order
- TheraFlu LAR menthol against viruses and sore throat, tablets N20Novartis
RUB 229 order
- TheraFlu LAR tablets against viruses and sore throat, tablets N16Novartis
182 RUR order
show more
Special instructions for the use of the drug Theraflu lar
The drug is not recommended for children under 4 years of age. The tablets should not be chewed. It is not recommended to take the drug immediately before or during meals to prevent food from entering the respiratory tract due to the local anesthetic effect of lidocaine. The spray can only be used on the mucous membrane (mouth and throat). Do not use the spray if the spray nozzle is damaged. During pregnancy and breastfeeding . Due to the lack of clinical data, the drug should be taken during pregnancy and lactation only when the benefits of use outweigh the potential risk to the fetus/child. Impact on the ability to drive vehicles and operate machinery. Does not affect.