Home | About us | Delivery | Advertisers | Login | Registration
Delivery on Sundays and holidays does not work!
- Medicines
- dietary supplementsVitamins
- Categories from A to Z
- Brands from A to Z
- Products from A to Z
- Medical equipment
- beauty
- Child
- Care
- Honey products appointments
- Herbs and herbal teas
- Medical nutrition
- Journey
- Making medicinesStock
Pharmacy online is the best pharmacy in Almaty, delivering medicines to Almaty. An online pharmacy or online pharmacy provides the following types of services: delivery of medicines, medicines to your home. Online pharmacy Almaty or online pharmacy Almaty delivers medicines to your home, as well as home delivery of medicines in Almaty.
my basket
Apteka84.kz is an online pharmacy that offers its customers medicines, medicinal and decorative cosmetics, dietary supplements, vitamins, baby food, intimate products for adults, medical equipment and thousands of other medical and cosmetic products at low prices. All data presented on the Apteka84.kz website is for informational purposes only and is not a substitute for professional medical care. Apteka84.kz strongly recommends that you carefully read the instructions for use contained in each package of medicines and other products. If you currently have any symptoms of the disease, you should seek help from a doctor. You should always tell your doctor or pharmacist about all the medicines you take. If you feel you need further help, please consult your local pharmacist or contact our GP online or by telephone.
© 2021 Pharmacy 84.
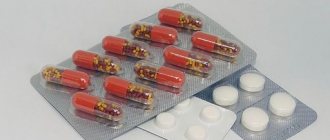
Aktiferrin caps No. 20
Active substance
Ferrous sulfate + Serine
Manufacturer
Merkle GmbH (Germany)
Shelf life
4 years
Storage conditions
At a temperature not exceeding 25 °C
Registration certificate number
P N015041/02 dated 11/18/2013
Compound
| Drops for oral administration | 100 ml |
| active substances: | |
| iron sulfate heptahydrate | 4.72 g |
| (corresponds to 0.948 g iron (II) | |
| D,L-serine | 3.56 g |
| excipients: ascorbic acid - 0.8 g; potassium sorbate - 0.1 g; invert sugar syrup (72.7% TS) - 15.18 g; ethanol (96%) - 0.1563 g; creamy flavoring - 0.0125 g; raspberry flavoring - 0.05 g; purified water - 83.4 g |
| Capsules | 1 caps. |
| active substances: | |
| iron sulfate | 113.85 mg |
| (corresponds to 34.5 mg iron (II) | |
| D,L-serine | 129 mg |
| excipients: lecithin (E322) - 10 mg; rapeseed oil - 142.15 mg; hydrogenated soybean oil - 7.5 mg; soybean oil, partially hydrogenated - 30 mg; yellow beeswax - 7.5 mg | |
| shell: gelatin - 161.64 mg; glycerol 85% - 40.23 mg; sorbitol - 26.27 mg; iron dye black oxide (E172) - 2.16 mg; red iron oxide dye (E172) - 0.9 mg |
Characteristic
| Drops for oral administration | 100 ml |
| active substances: | |
| iron sulfate heptahydrate | 4.72 g |
| (corresponds to 0.948 g iron (II) | |
| D,L-serine | 3.56 g |
| excipients: ascorbic acid - 0.8 g; potassium sorbate - 0.1 g; invert sugar syrup (72.7% TS) - 15.18 g; ethanol (96%) - 0.1563 g; creamy flavoring - 0.0125 g; raspberry flavoring - 0.05 g; purified water - 83.4 g |
| Capsules | 1 caps. |
| active substances: | |
| iron sulfate | 113.85 mg |
| (corresponds to 34.5 mg iron (II) | |
| D,L-serine | 129 mg |
| excipients: lecithin (E322) - 10 mg; rapeseed oil - 142.15 mg; hydrogenated soybean oil - 7.5 mg; soybean oil, partially hydrogenated - 30 mg; yellow beeswax - 7.5 mg | |
| shell: gelatin - 161.64 mg; glycerol 85% - 40.23 mg; sorbitol - 26.27 mg; iron dye black oxide (E172) - 2.16 mg; red iron oxide dye (E172) - 0.9 mg |
Directions for use and doses
Inside.
Iron deficiency can be approximately calculated using the formula: mg iron = kg body weight × 3.5 × (16 − Hb in g%).
The threshold values below or above which iron deficiency is considered to require treatment are indicated in the table.
Table
| Indicators | Children under 6 years old | Children over 6 years old and adults |
| Hb, g% | <11 | <12 |
| Red blood cells, million/mm3 | <3,5 | <4,0 |
| Reticulocytes, % | >15 | >15 |
| Serum Fe, mcg% | <60 | < 80 |
| Total iron content = transferrin, mcg% | >380 | >400 |
| Average erythrocyte hemoglobin, pg | <25 | <30 |
| Average red blood cell volume | <30 | <30 |
The duration of treatment depends on the etiology and severity of the disease.
To ensure an adequate response, iron therapy should last at least 8 weeks. With subsequent normalization of hemoglobin levels, treatment should be extended for the next 6–8 weeks to replenish the iron depot.
Monitoring indicators.
If necessary, the degree of iron deficiency and the subsequent need for iron replacement should be monitored by the following laboratory parameters at approximately 4-week intervals: hemoglobin, red blood cells, reticulocytes, serum iron, transferrin, mean erythrocyte hemoglobin, mean erythrocyte volume.
If the doctor has not prescribed other dosages, then you should strictly adhere to the instructions given below.
Drops for oral administration
The daily dose is set at the rate of 5 drops/kg, the frequency of administration is 2-3 times a day.
Infants: the average dose is 10–15 drops 3 times a day.
Preschool children: average dose - 25-35 drops 3 times a day.
School-age children: average dose - 50 drops 3 times a day.
To open the bottle, you need to press the cap down and at the same time turn it in the direction of the arrow. After using the medicine, install the cap and screw it tightly (prevents access for children).
Capsules
Aktiferrin capsules should be swallowed whole, without chewing, with a small amount of liquid. Take 30 minutes before meals, between meals, with fruit juice containing vitamin C to improve iron absorption.
Adults and adolescents weighing over 50 kg: 1 caps. 1–2 times a day.
Children weighing 20–50 kg: 1 capsule. 1 time per day.
Conditions for dispensing from pharmacies
On prescription.
OCEAN-AKT-INFblock-170914-MEDIA-649-160915
Pharmgroups
Iron supplement (Hematopoiesis stimulants in combinations)
Pharmaceutical actions
replenishes iron deficiency, hematopoietic
Actiferrin 30 ml drops for oral administration
Instructions for medical use of the drug Aktiferrin ® Trade name Aktiferrin ® International nonproprietary name No Dosage form Oral drops, 30 ml Composition 100 ml of the drug contains active substances: iron (II) sulfate hemihydrate 4.720 g, micronized D, L-serine 3.560 g , excipients: acesulfame potassium, ascorbic acid, sodium benzoate, hydrochloric acid 25%, polyethylene glycol 400, caramel coloring, blackcurrant flavoring, purified water. Description Transparent, brownish-yellow solution with the smell and taste of black currant Pharmacotherapeutic group Hematopoiesis stimulants. Iron supplements. Iron (II) preparations for oral administration. ATC code B03AA Pharmacological properties Pharmacokinetics After oral administration, the iron included in the drug Actiferrin® is quite completely absorbed from the gastrointestinal tract into the systemic bloodstream. The degree of absorption depends on the severity of iron deficiency (the greater the deficiency, the higher the absorption). The maximum concentration of iron in the blood serum is achieved within the first 2-4 hours after taking the drug. Excreted in feces, urine and sweat. Pharmacodynamics Iron is necessary for the life of the body: it is part of hemoglobin, myoglobin, and various enzymes; reversibly binds oxygen and participates in a number of redox reactions; stimulates erythropoiesis. The alpha-amino acid serine included in Actiferrin® promotes more efficient absorption of iron and its entry into the systemic circulation, leading to the rapid restoration of its normal content in the body. This ensures better tolerability of the drug and allows you to reduce the required dose of iron. Taking Actiferrin® drops stimulates erythropoiesis, normalizes hematological parameters, helps normalize the concentration of iron in the blood serum, which leads to a gradual regression of clinical (weakness, fatigue, dizziness, tachycardia, dry skin) and laboratory symptoms. Indications for use - treatment and prevention of iron deficiency anemia. Method of administration and dosage Actiferrin® is taken orally immediately before meals or during meals with a small amount of liquid (fruit tea or water). If your doctor has not prescribed other dosages, you should strictly adhere to the instructions given below. Aktiferrin® drops are the most convenient form for newborns and young children: The daily dose is set at the rate of 5 drops per 1 kg of body weight, the frequency of administration is 2-3 times a day. Infants: 10-15 drops 3 times a day. Preschool children: 25-35 drops 3 times a day. School-age children: 50 drops 3 times a day. After achieving normal hemoglobin levels, treatment with Actiferrin® is continued for at least 8-12 weeks. Side effects Actiferrin® Drops are usually well tolerated by patients of any age. Often - nausea, feeling of heaviness in the epigastric region, flatulence, constipation or diarrhea, disappearing when the dose is reduced or when taking the drug with food Rarely - allergic skin reactions Contraindications - hypersensitivity to the components of the drug - hemochromatosis, hemosiderosis - other types of anemia not caused by deficiency iron in the body (for example, sideroachrestic anemia, anemia due to lead poisoning, thalassemia, hemolytic, megaloblastic, aplastic anemia) Drug interactions When prescribing the drug Actiferrin® and: - tetracyclines, gyrase inhibitors (for example, ciprofloxacin, levofloxacin, norfloxacin, ofloxacin), bisphosphonates, penicillamine, levodopa, carbidopa and methyldopa - their absorption is reduced; — thyroxine – absorption of thyroxine decreases; - zinc - absorption of zinc decreases; - vitamin E in children - the effectiveness of vitamin E decreases. - cholestyramine, antacids (containing aluminum, magnesium, calcium or bismuth), supplements containing calcium and magnesium - iron absorption decreases; - non-steroidal anti-inflammatory drugs - the irritating effect of iron on the mucous membrane of the gastrointestinal tract may increase. It is recommended to take the above medications 2-3 hours before taking Actiferrin®. Vitamin C or citric acid increases iron absorption. Glucocorticoids may enhance the stimulation of erythropoiesis by Actiferrin®. Special instructions With caution Caution should be exercised when using iron supplements together with dietary products and supplements containing iron salts (possible risk of overdose). In patients with inflammation and ulcers of the gastrointestinal mucosa, the balance between the benefits of treatment and the risk of developing exacerbations of gastroenterological diseases during iron therapy should be assessed. When prescribing to patients with diabetes, it should be borne in mind that 1 ml of drops contains 64 mg of glucose, which is equivalent to 0.0053 bread units. If necessary, the degree of iron deficiency and subsequent need for iron replacement should be monitored by the following laboratory parameters at approximately 4-week intervals: hemoglobin, red blood cells, reticulocytes, serum iron, transferrin. A ferritin level of less than 15 μg/L indicates a lack of iron stores in the body. To prevent the appearance of reversible dark plaque on patients' teeth, Actiferrin® drops should not be taken undiluted, and after eating it is recommended to thoroughly brush the teeth. Aktiferrin® drops should not be taken with black tea, coffee, or milk to avoid decreased iron absorption. In addition, a decrease in absorption can be caused by: solid foods, bread, raw cereals, dairy products, eggs. When taking Aktiferrin® drops, stool turns black, which is not clinically significant. Pregnancy and lactation Preclinical studies on reproductive function have not been conducted. Use during pregnancy and lactation is possible if the potential benefit to the mother outweighs the potential risk to the fetus and child. Peculiarities of influence on the ability to drive a vehicle and potentially dangerous mechanisms Does not affect the ability to drive vehicles or other moving mechanical equipment. Overdose Children have a high risk of intoxication with iron preparations; life-threatening conditions can occur when taking 1 g of ferrous sulfate. Therefore, iron supplements should be stored out of the reach of children. Symptoms: nausea, abdominal pain, diarrhea, vomiting, in severe cases - cyanosis, confusion, symptoms of hyperventilation, after 12-48 hours, shock with Cheyne-Stokes breathing, oliguria, jaundice due to toxic hepatitis, toxic liver failure is possible . In some cases, central nervous system disorders such as paralysis, convulsions, coma, and bleeding disorders. Treatment: before transportation to the hospital - take milk, raw eggs. Before carrying out specific therapy, measures to remove the drug from the gastrointestinal tract (inducing vomiting, gastric lavage with bicarbonate and phosphate solutions), symptomatic treatment of shock and acidosis. Specific therapy is carried out at a serum iron concentration of 300-350 mcg/dl by prescribing deferoxamine (Desferal) 1-2 g intravenously at a rate of 15 mg/kg body weight per hour every 3-12 hours. In case of acute poisoning, to bind iron that has not yet been absorbed from the gastrointestinal tract, 5-10 g of the drug is given orally by dissolving the contents of 10-20 ampoules in drinking water. Patients with oligo and anuria with an overdose of iron salts are prescribed peritoneal or hemodialysis. Release form and packaging 30 ml in brown glass dropper bottles with a screw cap. 1 bottle, along with instructions for medical use in the state and Russian languages, is placed in a cardboard box. Storage conditions Store out of the reach of children at a temperature not exceeding 25°C. The period of use after the first opening is 1 month. Shelf life: 2 years Do not use after expiration date. Conditions for dispensing from pharmacies By prescription, Germany Owner of the registration certificate "ratiopharm GmbH", Germany Address of the organization that accepts claims from consumers on the quality of products (products) in the territory of the Republic of Kazakhstan "ratiopharm Kazakhstan" LLP, Almaty, Al-Farabi Ave. 19 , BC "Nurly-Tau", 1B, office. 603, tel. (727)3110915, fax: (727)3110734, e-mail