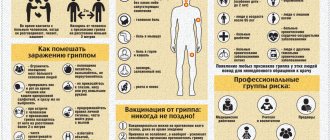
Prevention of influenza, ARVI and coronavirus is a single set of measures aimed at preventing morbidity. The words of Hippocrates are relevant today: “It is easier to prevent a disease than to treat it.” Compliance with preventive measures helps protect against infection, reduce the risk of large-scale spread of infection, and save people’s lives.
With the advent of the pandemic, the whole world has felt the importance of preventing respiratory infections. COVID-19 has forced humanity to take a fresh look at ways to stay healthy. On a par with coronavirus infection are influenza and ARVI, which people encounter in the fall and spring. As practice shows, seasonal diseases sometimes cause complications no less severe than Covid. Prevention of influenza and ARVI, developed by epidemiologists, helps protect against infection and its consequences. Read about the characteristics of respiratory viruses, means and methods of protection against infection in our article.
Ways to get a respiratory infection
Respiratory viruses are a large family of ultramicroscopic intracellular parasites. Some of them become noticeably more active during the demi-season, causing epidemics of ARVI (acute respiratory viral infection). These are paramyxoviruses, picornaviruses, adenoviruses, coronaviruses, parainfluenza viruses, influenza A, B, C.
Pathogens of infectious respiratory diseases are highly contagious (infectious), but they can only enter the body through the mucous membranes of the respiratory tract. The main method of infection is aerogenic or airborne droplets. Viral particles fly through the air with droplets of liquid that escape from the mouth of a sick person when sneezing or coughing. The second method of infection is through household contact. The direct route is through a handshake with a carrier of the infection, the indirect route is through contaminated (infected) surfaces. Having picked up the virus in your hands, it is easy to carry it into your nose, your mouth is the “entrance gate” of ARVI.
Chronic hepatitis C is a completely curable disease
The hepatitis C virus remains the leading cause of chronic hepatitis and liver cirrhosis, as well as primary liver cancer in the world, including in Russia, which ranks sixth in the number of patients with chronic hepatitis C. Over the past 25 years, significant progress has been made in the treatment of chronic hepatitis C. It has been proven that in case of viral hepatitis C, as a result of antiviral therapy, complete elimination of the virus from the human body . This means that after successful treatment, the virus no longer returns, as a result, inflammation in the liver stops , and the disease is completely cured . It is important that treatment at the stage of hepatitis eliminates the risk of developing liver cirrhosis and life-threatening complications, including liver cancer. In cases where the viral infection is eliminated at the stage of liver cirrhosis, the risk of developing liver cancer is significantly reduced, although it is not completely eliminated. Therefore, chronic hepatitis C must be treated as early as possible.
Means for the prevention of acute respiratory viral infections and influenza
Preventive measures against acute respiratory infections are divided into specific and nonspecific. Specific prevention is vaccination. The Russian Federation is immunizing the population against influenza and coronavirus. There is no vaccine for other viruses, of which medical virology numbers more than two hundred. Non-specific methods include drug protection of the body, home remedies to strengthen the immune system, rules for the prevention of influenza and ARVI, recommended by WHO.
Nootropic drugs with proven effectiveness
Nootropics is a broad concept that includes any drugs that specifically affect higher mental functions.
Here we have memory, attention, learning ability, and concentration. Sometimes other related remedies are included here, for example, to combat desynchronosis (jet lag) or kinetosis (motion sickness). At the same time, the evidence base for nootropics is quite clearly divided into two parts. Everything that concerns the use of such drugs by obviously healthy people for the purpose of “biohacking”, that is, improving the body’s capabilities beyond the existing ones, here we can safely say that there are no proven effects and consider nootropics to be dummies, at best - level D. In the same case, When it comes to patients with cognitive decline, the results can range from A to C on the evidence scale.
On the other hand, if we consider caffeine and nicotine as nootropics, then they demonstrate very good effects even in meta-analyses, while caffeine short-term increases activity and attention, and nicotine, also short-term, improves concentration, episodic and working memory, and also slightly improves fine motor skills .
Nonspecific prevention
To prevent influenza and ARVI, two types of pharmacological drugs have been developed:
• Direct acting drugs. They block enzymes on the surface of the virus. Because of this, the infectious agent loses the ability to reproduce its RNA and proteins. • Agents that stimulate the production of protective interferons to increase the body's resistance to infections.
Antiviral medications are available in the form of tablets, capsules, and powders. For children, there are syrups, suspensions, and drops that are convenient to take.
The TOP 5 drugs include:
• Arbidol. Active against most pathogens of acute respiratory viral infections, including influenza viruses. For prevention, take twice a week, for treatment - 4 times a day. In powder form it is allowed for children from 2 years old, in tablet form - from 6 years old. • Ergoferon. A fast-acting immunomodulator with proven effectiveness against respiratory viruses. Tablets for sublingual administration are prescribed: 5 pcs per day. within 2 hours, on subsequent days - 1 pc. three times. • Ingavirin. An innovative antiviral agent with high anti-inflammatory activity. Used for all types of acute respiratory viral infections, including coronavirus infection. In pediatrics it is used from 7 years of age. To achieve results, 1 capsule per day is enough. • Kagocel. A powerful immunostimulant, it causes the synthesis of interferons by all cells of the body that participate in the antiviral response. Allowed for children from 3 years old. Prevention is carried out in cycles: 2 days, 2 tablets - 5 days break - 2 days, 2 tablets. • Anaferon. Increases the production of interferon, prescribed for the prevention and treatment of acute respiratory viral infections. In pediatrics, a specially developed children's version of the drug is used.
Unlike antibiotics, antivirals are sold without a doctor's prescription.
Organization of home prevention of ARVI and influenza includes:
• Nutrition correction. Foods high in ascorbic acid—vegetables, wild berries, fruits, herbs—help stimulate the formation of interferon. Protein is the building material for immune cells, so the diet should contain a sufficient amount of meat, fish, dairy products, and cereals (millet, oats, buckwheat). The main helper of immunity is vitamin D. It is found in egg yolk, seaweed, and cod liver. • Hardening, physical activity. It is recommended to start hardening procedures in the summer - before the start of seasonal epidemics. Sports activities saturate the blood with oxygen, accelerate hemodynamics, and increase blood supply to organs. This allows you to maintain the functionality of immune system cells to produce interferons.
• Use of folk remedies. Products with antimicrobial effects (garlic, ginger, onions), as well as plants with immunostimulating properties (ginseng root, rose hips, echinacea, sage, lemon balm) help protect against seasonal diseases.
To prevent illness, it is important to adhere to a rational work and rest regime. Doctors recommend getting enough sleep, walking daily, avoiding conflict situations that lead to stress, and taking vitamin and mineral supplements.
Prevent severe disease. How does the cure for COVID-19 work?
In the updated recommendations of the Ministry of Health for the diagnosis and treatment of COVID-19, the antiviral drug favipiravir is first mentioned - in Russia it is produced under the names “Areplivir”, “Coronavir” and “Avifavir”. Judging by clinical trials here and abroad, it effectively combats the multiplication of the virus in the body, shortens the treatment period and is suitable for outpatients. However, local doctors are skeptical about the new medicine and are in no hurry to prescribe it. RIA Novosti, together with experts, understands how the drug works and why medicine gives it the green light.
What Japanese scientists found out
With the onset of the COVID-19 pandemic, scientists immediately began to look for a remedy against the causative agent of the disease. Creating a specific medicine from scratch is a long process; it will take years, so we analyzed existing ones.
One candidate was favipiravir, developed by the Japanese company Toyama Chemical in 2002 as a treatment for influenza. It disrupts the reproduction processes of RNA-containing viruses, which include SARS-CoV-2, and triggers the processes of their self-destruction in the body. In test mode, the drug was given to patients with COVID-19.
A preliminary report from an observational program in Japan conducted in May on more than 2,000 people found that those taking favipiravir felt better as early as the seventh day of treatment. Positive dynamics were observed in 73.8% of mild patients, 66.6% of moderate patients and 40.1%, among whom were people with cardiovascular problems, diabetes and chronic lung disease.
A total of 39 clinical trials of favipiravir have been registered worldwide. It has been approved for treatment in China, India, and Thailand. It is also recommended in Russia. However, medical practitioners have mixed opinions about the drug.
Doubts and evidence
“They treat it differently - some strictly follow the recommendations of the Ministry of Health, others do not prescribe it at all, because they are not too confident in its effectiveness,” notes Pavel Nikitin from the N. N. Burdenko National Medical Research Center for Neurosurgery.
According to him, favipiravir did demonstrate antiviral activity against other RNA viruses in preclinical studies. But in order to definitely recommend it, deeper and more detailed research is needed according to the standards of evidence-based medicine. “Scientific data, in my opinion, is still not enough,” says the scientist.
Dmitry Rosukhovsky, a vascular surgeon and chief physician at the Lastikmed clinic in St. Petersburg, is also skeptical: “Already a year has passed since the start of testing potentially reliable remedies for coronavirus. And everyone is not efficient enough.”
Wariness towards favipiravir, he believes, is explained by negative experience with the use of other drugs that affect the cause of the disease.
Doctors who tested favipiravir-based drugs in the clinic have a different opinion.
“We deal with Areplivir as soon as it appeared. The experience of use was gained from the staff of the Department of Infectious Diseases - they were the first to admit patients with COVID-19. Since September we have been monitoring data from the regional hospital. We have an idea of how the drug works from both a practical point of view and a scientific one, since colleagues from the university participated in clinical trials,” says Candidate of Medical Sciences Lyudmila Tverdova, an endocrinologist of the highest category, associate professor at the Ryazan State Medical University named after Academician Pavlov.
During the randomized trial, patients were given antiviral drugs or standard therapy drugs recommended by the Ministry of Health. It turned out that those who received Areplivir as part of complex treatment spent less time in the hospital and got back on their feet faster.
“Ideally, clinical trials take years to plan, recruit hundreds of patients, and look at targets for molecules to act on. But we have a unique situation. There is not a single drug developed specifically for COVID-19. Medicines for malaria, despite the side effects, were used in practice in our country. Favipiravir has never been used in Russia. Yes, it is registered in Japan, and many scientific articles describe its successful action against various RNA viruses - say, influenza or even Ebola. But Russian doctors are not yet very familiar with it, which largely explains their skepticism. The only way is clinical research,” says Kira Zaslavskaya, national medical manager of Promomed LLC, which produces Areplivir.
Medicines for COVID-19 are being tested using an accelerated procedure. This reduces bureaucratic difficulties, Zaslavskaya notes, but does not detract from the value of the results, which in any case should have statistical significance. The Areplivir study involved 206 people, and the Coronavir study - 168. For the third trial, 540 participants were announced, the results have not yet been published. “All data, both Russian and international, goes into the piggy bank. “Soon we will receive the highest level of evidence of the results of clinical studies of favipiravir,” the expert emphasizes.
"Effective in the early stages"
“We noticed that the drug Areplivir is more effective in the first five days of the disease, in the early stages. In our opinion, it is better to use it on an outpatient basis, and not just in a hospital, where patients are admitted with moderate and severe forms. In addition, it reduces the effectiveness of infection,” Lyudmila Tverdova shares her observations.
The fact that medications based on favipiravir can be used not only in hospitals, but also in outpatient settings is also stated in the temporary recommendations of the Ministry of Health.
“To reduce the risk of severe disease and, accordingly, the burden on the healthcare system, it is necessary to use effective drugs. Favipiravir acts directly on the virus reproduction mechanism, and now it is the only one. In the Areplivir study, which was conducted on moderately ill patients, after a course of administration, 98% of patients experienced elimination of the virus. This was demonstrated in a study by our colleagues,” says Zaslavskaya.
Antiviral therapy is needed to prevent a mild form from becoming moderate, and a moderate form from becoming severe. Of course, sooner or later the elimination of the virus occurs on its own. But if by this moment there are large-scale lesions and hyperactivation of the immune system, then the risk of serious complications is very high, explains a representative of Promomed.
The company will soon launch observational studies of the use of Areplivir in routine clinical practice. Doctors will receive new data on the safety and effectiveness of the drug.
Effective, but affordable?
So, manufacturers and researchers of favipiravir are optimistic, but until recently it was distributed only in hospitals; it was almost impossible to buy at retail even in Moscow. In the regions there are still problems with ordering through online services - medicines are simply “out of stock”.
Now the situation has begun to change. The drugs appeared in pharmacies, but only in those connected to the digital labeling system introduced on July 1 of this year in Russia. Now the manufacturer applies a unique QR code to each package so that it can be tracked at every stage from the conveyor to the consumer. Distributors and pharmacy chains must read the label and enter it into the federal database - IS MDLP.
In practice, all this resulted in chaos: the system regularly crashed, documents were not processed for a long time, calls to technical support remained without resolution. In fact, there is no shortage of medicines; often they are already in the supplier’s warehouse, but they cannot be sold. “The problem also affected medications used to treat covid infection; in some companies, the circulation of these drugs has been suspended,” the press service of the Association of Russian Pharmaceutical Manufacturers (ARPP) explained to RIA Novosti.
Officials have received many complaints and requests regarding the work of the new labeling system from both manufacturers and pharmacy chains. Only ARFP sent three letters to M.V. Mishustin, V.I. Matvienko, V.V. Volodin, where they proposed “to establish a special procedure for working with IS MDLP during the COVID-19 epidemic in order to ensure the unimpeded movement of products in the supply chain.” As a result, on October 27, the Ministry of Industry and Trade met halfway and simplified the procedure for working with the system.
Now the drug costs about 5,500 rubles per package. Often this is enough for a course of treatment, because the main task is to stop the spread of the virus. Self-medication is unacceptable - the medicine is dispensed only as prescribed by a doctor.
“Outpatient patients can purchase the drug using prescription form number 107 (not strict reporting) from a local physician, or a pulmonologist, or a general practitioner. There shouldn’t be any difficulty in getting a prescription,” explained Lyudmila Tverdova.
However, favipiravir drugs will be provided free of charge to those receiving treatment at home. Moscow is already doing this, and the regions are next. The government recently decided to allocate five billion rubles for this purpose. Funds have been transferred to the constituent entities of the Federation, and procurement has begun. “By the end of the year, at least 640,500 patients will be provided with free medicines from the federal budget, excluding the city of Moscow,” says the official statement of the Russian Ministry of Health.
As RIA Novosti was told by Promomed LLC, the plant has doubled its capacity, which is enough to meet the needs of the regions, and is now actively shipping goods.
Flu and its dangers
Besides coronavirus, the most dangerous causative agent of acute infections is the influenza virus. It differs from its respiratory “relatives” in its high virulence - its ability to quickly spread throughout the body, causing severe complications - even lethality. The risk group includes people of advanced age, young children, patients with chronic cardiac and pulmonary pathologies.
The influenza virus provokes infectious and inflammatory diseases of the heart, nervous system, and brain. With a heavy viral load, intoxication quickly develops, the permeability of the vascular walls increases, hemodynamics are disrupted, the lungs lose their ability to absorb oxygen, and respiratory failure occurs, which can be fatal. The most effective prevention of influenza is the introduction of an influenza vaccine before the start of the epidemic season. Children are vaccinated from 6 months of age.
Interferon-alpha drugs are becoming a thing of the past...
In recent years, approaches to the treatment of chronic hepatitis C have changed significantly, as a result of which it has become possible to achieve the highest possible effectiveness.
Let us recall that since 2000, for several years, the “gold standard” has been combination therapy with two drugs - pegylated interferon-alpha (injected once a week) and ribavirin (oral tablets). This treatment made it possible to eliminate the virus and cure the disease in 80-90% of patients with genotypes 3 and 2 of the virus and about 50% with genotype 1. The effectiveness of treatment largely depended not only on the genotype of the virus, but also on the stage of fibrosis (effectiveness in treatment of compensated liver cirrhosis was unsatisfactory), as well as on individual sensitivity to interferon-alpha, which was determined using a genetic study of polymorphism of the interleukin 28B gene. Thus, this therapy was not effective enough for the most common genotype 1 of the C virus in Russia, especially in patients with liver cirrhosis, patients with an unfavorable genotype of interleukin 28B, as well as patients with hepatitis C, post-transplant patients and those infected with HIV. In addition, the therapy was long-term (for genotype 1 - 48 weeks) and associated with a deterioration in well-being and quality of life during the treatment period. A significant proportion of patients could not receive this therapy due to the presence of other diseases that are a contraindication to the use of interferon or ribavirin due to the high risk of complications during treatment.
Since 2011, the first drugs with direct antiviral action have appeared abroad and then in Russia - virus C protease inhibitors (telaprevir, boceprevir, then simeprevir), the use of which for genotype 1 in combination with interferon-alpha drugs and ribavirin (the so-called “triple” combination antiviral therapy) significantly increased the effectiveness of treatment. However, this therapy was still associated with a risk of complications during treatment, especially in patients with liver cirrhosis, in whom the effectiveness of treatment remains significantly lower than in patients with hepatitis.
In recent years, new direct antiviral drugs have appeared abroad that attack the hepatitis C virus at various stages of its life cycle and stop its reproduction. Combinations of such drugs make it possible to achieve high efficiency (close to 100%) with good tolerability of treatment and a reduction in its duration. Clinical studies have shown the high effectiveness of a number of combinations consisting of 2-3 drugs with direct antiviral action. Some of these combinations are applicable only for 1 genotype of the virus, others - for all genotypes of the virus. It is important that interferon-free treatment regimens are highly effective at the stage of liver cirrhosis. In combination with drugs with direct antiviral action, the use of ribavirin remains important in some patients. Interferon-alpha drugs, which until recently represented the basis of antiviral therapy for chronic hepatitis C and created the greatest problems of treatment tolerability, are becoming a thing of the past.
Vitamins to strengthen the immune system
Special blood tests help determine which vitamins the body lacks. The main marker of stable immune function is considered to be normal levels of vitamin D hormone. It enters the body with food and is synthesized by skin cells under the influence of sunlight. Considering the deficiency of ultraviolet radiation in most Russian regions, it is recommended to take the vitamin hormone in the form of a dietary supplement. To prevent respiratory diseases, the immune system needs B vitamins, ascorbic acid, and fat-soluble vitamins A and E.
Products that contain vitamins for the prevention of ARVI and influenza
Ascorbic acid (vitamin C):
- sauerkraut
- bell pepper
- citrus
- cranberry
- currant
- rose hip
Group B:
- nuts
- milk
- legumes
- buckwheat
- oats
- tomatoes
Tocopherol (E):
- sea buckthorn
- spinach
- broccoli
- bran
- eggs
- vegetable oils
Retinol (A):
- carrot
- green onions
- parsley
- peaches
- apricots
- melon
Replenishing vitamin reserves through nutrition alone is an impossible task. To maintain the immune system in a state of combat readiness, an additional source is needed - pharmacy vitamin complexes:
• Supradin Immuno Forte; • Doctor More; • Multi-tabs Immuno Plus; • Pediakid syrup; • Superum Echinacea Vitamin C Complex.
The most modern and effective means for strengthening the immune system can be ordered with home delivery.
Evidence-based medicine and clinical research
The first criticism of the misuse of statistical methods in medical research was published in 1835 in the French scientific journal Comtes Rendus de l'Académie des Sciences.
However, for a long time it was not possible to formulate the basic principles: how to organize the study of drugs, what they should be aimed at. One of the founders of what is now commonly called evidence-based medicine
, is considered to be Archibald Cochrane (1909-1988), a Scottish physician whose book Efficiency and Effectiveness: Random Reflections on Health Care was the first important work in a new direction.
Cochrane formulated the main idea as follows: “... since resources will always be limited, they must be used to ensure equitable provision of those forms of health care that have been shown to be effective in well-designed studies evaluating their effectiveness.”
From a modern point of view, evidence-based medicine is the use of the best clinical research results to select treatment for a particular patient, integrating the best scientific evidence with clinical experience and patient expectations. This definition is given in the Russian textbook “Fundamentals of Evidence-Based Medicine” in 2010, edited by Academician R.G. Oganov. To put it simply, evidence-based medicine is based on three pillars: scientifically based data, clinical thinking and, no matter how strange it may sound, common sense.
The evidence-based approach is based on clinical research
medications, during which their effectiveness is tested. True, it all starts a little earlier, with research laboratories. There, scientists find millions of candidate molecules, which are then subjected to rigorous selection using a variety of biochemical methods.
As a result, only a small number of them proceed to the preclinical research
.
The first step is to ensure that the candidate molecule does not kill a living cell. To do this, a series of experiments are carried out on cell cultures, that is, in vitro
, “in glass”. There will likely be at least two options: a rapidly proliferating line of tumor cells to quickly track changes over generations, as well as a line of specialized cells that are targeted by a future drug.
The next important milestone in preclinical research is experiments on laboratory animals, in vivo.
. Cells are cells, but we need to make sure that dangerous metabolites are not formed, that the drug does not affect the developing fetus or future generations, that it works as its developers intended, and that the side effects are significantly less pronounced than the main ones.
And only when preclinical studies are completed can we move on to people, that is, begin clinical studies
(CI). An important nuance. CT can be deciphered as both clinical research and clinical trials. Both options are legitimate and can be used both in scientific literature and in ordinary conversation.
Clinical research is defined by the International Committee of Medical Journal Editors. In particular, it states that a clinical trial is any research project in which participants are prospectively divided into experimental and control groups to study the cause-and-effect relationship between a medical intervention and the human body's response to it.
Each clinical trial is conducted in three mandatory phases. During Phase I
are studying so-called acute toxicity in humans. We have identified this parameter in cell cultures and mice, but confirmation is needed in human volunteers. For this reason, in phase I, healthy participants are recruited, during which a large package of documents is prepared, including detailed insurance. Often such work is paid - people still take risks by testing a new drug on themselves. As a result, researchers get an answer to the question: “Is the new drug safe?” Usually there are few participants in phase I, 30-50 people. In addition to direct toxicity, such an important issue as side effects is also being studied. Sometimes they can be a reason to stop a clinical trial if the side effects are more pronounced than the main effect, and sometimes to change the drug profile; a classic example is sildenafil, which was initially studied as a drug against pulmonary hypertension and only later, when phase I participants began to report very As an interesting side effect, the clinical trial was repurposed to study the first drug against erectile dysfunction.
After successful completion of this stage, they proceed to phase II
KI. There are already more participants here - several hundred, and here patients with the disease against which the new drug should work can be recruited as volunteers. The main question that interests phase II researchers is: “Does the new drug work?” An important feature of phase II is that a comparison group appears here for the first time. We compare the clinical effectiveness of the study drug with a placebo, which is a dummy drug that looks exactly like the study drug, but neither the participating patients nor the doctors working with them know which group the patient is in. This is necessary for maximum correctness of the results obtained. Also, during phase II, the collection of information on side effects continues; dosage and frequency regimens can be studied.
If this stage of the drug is successful, the longest and largest part of the clinical trial begins - phase III a
. The number of participants here is already in the thousands, and sometimes tens of thousands. Thus, in phase III studies of each vaccine against COVID-19, from 25 to 40 thousand people took part. Here again a comparison is made, and researchers are interested in the question: “How does the new drug perform compared to those already existing on the market?” If this is a completely new drug, it can be compared with a placebo in the third phase. Questions are also being studied: is the drug effective with long-term use, what side effects are observed after 3-6-9-12 months of use, how long does the main effect last? Economic issues are also studied. So, if a new product does not differ in its main and side effects, but is cheaper than existing drugs, it has every chance of entering the market.
Typically, the completion of each phase is accompanied by the collection and analysis of the data obtained, as well as publication in one of the scientific medical journals. All information received, and this is a huge array, including data from all participants in all phases, is collected in a so-called registration dossier and transmitted to the regulatory supervisory authority, for example, the US Food and Drug Administration, the European Medicines Agency, the Ministry of Health RF. If the provided documents meet the requirements of current regulatory documentation, the drug receives a registration certificate and marketing authorization, which opens the way to pharmacies and hospitals.
Thus, only one that has passed all stages: from preclinical studies to phase III clinical trials, and has received the appropriate approvals from the regulatory authority, can be considered a drug that has undergone clinical trials. It should be noted that often after entering the market, the IV, post-registration phase of a clinical trial
, when the manufacturer monitors the situation, observes how the new drug behaves in a huge population and may even recall it from pharmacies if something negative turns out.
What to do if you get sick?
Fever, weakness, soreness, lacrimation are early signs of infection. When they appear, it is not recommended to leave the house. Firstly, so as not to spread viruses, and secondly, so as not to worsen your health. You can call a therapist or pediatrician by phone. In case of extreme temperatures, it is better to call an ambulance. Before the doctor arrives, the sick person must be put to bed and given warm tea (fruit juice). It is advisable to put a medical mask on the patient, provide him with a separate room, dishes, and hygiene items. To prevent complications, it is important to follow medical prescriptions - take medications, drink plenty of fluids, ventilate the room, eat foods rich in vitamins, phytoncides, and protein. In the first days of illness it is necessary to observe bed rest. If you have the flu, physical activity can trigger the development of acute respiratory distress syndrome, an extremely life-threatening condition.
Are there any limitations and disadvantages of using interferon-free treatment regimens?
Thus, interferon-free regimens for the treatment of chronic hepatitis C differ from interferon-alpha-containing regimens in higher efficiency and safety, as well as a shorter duration of therapy and ease of use.
With the introduction of interferon-free treatment regimens, significant groups of patients have a chance to be cured in whom interferon-alpha-containing therapy had contraindications (these are patients with various autoimmune diseases, other severe concomitant pathologies) or was carried out but was ineffective (this group contains “difficult” to treat patients, including patients with liver cirrhosis).
The use of interferon-free treatment regimens has virtually no restrictions, with the exception of cases of incompatibility of direct antiviral drugs with certain medications taken by the patient for another disease, if these medications cannot be replaced or temporarily discontinued.
It is also important to know that some drugs with direct antiviral action are eliminated from the body primarily by the kidneys and may have limitations in their use in patients with severe renal failure, while some are eliminated primarily by the liver, which limits their use in patients with severe liver failure.
The choice of the optimal treatment regimen, assessment of drug interactions, as well as determination of the duration of treatment and the advisability of combining therapy with ribavirin are determined by a hepatologist.
Two groups of antiviral drugs
According to experts from the Food and Drug Administration (USA), only drugs that have a direct direct effect on the reproduction of the virus can be called antiviral, i.e., the action of these drugs is aimed at destroying the virus and preventing its reproduction.
According to many scientists, all currently existing drugs that fight viruses can be divided into two groups: drugs that act on the viruses themselves, and drugs that activate the body’s own immune defense.