Gout is a disease consisting of a disorder of uric acid metabolism.
Gout is usually classified as a joint disease because excess uric acid forms salts (urates), which are deposited primarily in the joints. The first joint affected is the joint of the big toe. An inflamed bump on the thumb is a characteristic symptom of gout. Genetically, men are more predisposed to this disease. Among the sick, almost 95% of them are. The peak incidence in men occurs at the age of 40-50 years, in women – from 60 years and older. However, gout can also affect people at a younger age.
General information about the disease
Gouty arthritis is a systemic metabolic disease associated with the deposition of uric acid salts in articular and periarticular tissues. Men get sick more often after 40 years of age. In women, gout is less common and begins later - in the postmenopausal period after 50-60 years. In total, gout affects about 2.5% of these populations. With age, this percentage increases significantly and by the age of 80 it is 9% in men and 6% in women. The ICD-10 code is M10.
Gouty arthritis is comorbid (often combined) with kidney diseases and chronic renal failure, cardiovascular diseases (angina pectoris, high blood pressure), type 2 diabetes mellitus, and obesity.
Despite the fact that treatment for this disease has been developed, it is not always possible to keep gout attacks under control. This mainly occurs due to patients’ misunderstanding of the mechanism of development (pathogenesis) of gout and refusal or irregular maintenance therapy.
Read more about arthritis, its symptoms and treatment in this article.
Causes of gout
- Taking medications:
thiazide diuretics, aspirin (2 g per day), cyclosporines. - Diseases leading to the appearance of gout symptoms:
coronary heart disease (CHD), arterial hypertension, metabolic syndrome, chronic renal failure, psoriasis, some blood diseases. The development of gout can also be promoted by organ transplantation and the introduction of a contrast agent during X-ray examinations. - Abuse of foods rich in purine bases can provoke and aggravate the development of this disease:
fatty meats and fish, alcohol, carbonated drinks, legumes, eggs, chocolate, mushrooms.
Cause and mechanism of development (etiology and pathogenesis) of the disease
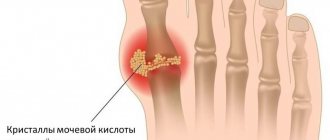
The cause of gouty arthritis is a disorder of purine metabolism. Purines are chemical compounds that form the basis of nucleic acids necessary for the formation of DNA and RNA molecules. As cells break down, purines are broken down into uric acid (UA). The latter enters the intercellular space and into the blood plasma, where it combines with sodium, forming a salt - monosodium urate (MUN).
An increased level of urate in the blood (hyperuricemia - GU) may be a consequence of a genetic predisposition (the kidneys do not eliminate MUN completely), high blood pressure (BP), consumption of large amounts of animal food, and alcohol.
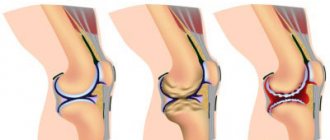
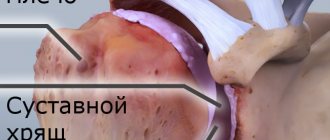
With an excess of urates, when they can no longer dissolve in the surrounding fluid (EOR concentration more than 0.4 mmol/l), the salts crystallize, deposit in the articular and periarticular tissues and are surrounded by protein rings. This formation is called tophi. The release of MUN from the tophi causes an immunological reaction: a large number of neutrophils (one of the types of leukocytes responsible for cellular immunity) appear in the synovial membrane and joint fluid.
Neutrophils ingest MUN crystals, which causes the release of proinflammatory (inflammation-causing and maintaining inflammation) cytokines and the development of an acute inflammatory response in the synovium. Acute attacks in the form of synovitis are very painful, but do not leave any consequences. The long-term chronic course of the disease with frequent repeated attacks leads to the destruction of articular cartilage, proliferation of bone tissue, deformation and dysfunction of the joint. Deposition of MUN in the kidneys causes a decrease in their function.
Factors contributing to increased urate levels in the blood:
- the presence in the diet of a large number of meat dishes, offal, eggs, alcoholic beverages,
- overweight;
- taking certain medications - diuretics, aspirin, nicotinic acid, medications to lower blood pressure (Concor), etc.;
- lead poisoning;
- increased breakdown of purines in blood diseases, psoriasis, etc.;
- increased formation of purines;
- impaired renal function and uric acid excretion.
Recommendations for antihyperuricemic therapy
The goal of antihyperuricemic therapy is to prevent the formation and dissolution of existing monosodium urate crystals by maintaining uric acid (UA) levels below 360 µmol/L.
- Allopurinol
– promotes adequate long-term antihyperuricemic therapy. The drug is recommended at a dose of 100 mg daily, if necessary, the dose is increased by 100 mg every two to four weeks. Patients with renal failure require dose adjustment of this drug.
- Uricosuric agents
(probenecid, sulfinpyrazone) are used as an alternative to allopurinol in patients with normal renal function. These drugs are relatively contraindicated in patients with urolithiasis.
- Benzbromarone
- powerful uricozourik; the drug is more effective than allopurinol. It is used for moderately reduced renal function, but requires monitoring due to hepatotoxicity.
- Colchicine
can be used as a prophylaxis for joint attacks during the first month of antihyperuricemic therapy (0.5-1.0 grams per day) and/or NSAIDs.
It is worth noting that in patients with gout, diuretics should be discontinued if possible (except for cases where diuretics are prescribed for health reasons).
- Losartan and fenofibrate
have a moderate uricosuric effect. These drugs are recommended for use in patients who are resistant to or intolerant of allopurinol or other uricosurics, in the presence of hypertension or metabolic syndrome. However, the clinical significance of such therapy and its cost-effectiveness are still unknown.
At the Clinic of High Medical Technologies named after. N.I. Pirogov patients will be able to determine the serum level of uric acid and other important biochemical blood parameters, as well as undergo clinical blood and urine tests, and receive qualified advice from a rheumatologist on treatment both during the interictal period of the disease and during the attack of an acute gouty arthritis.
Classification
Based on the causes of occurrence, gouty arthritis is divided into forms:
- primary
– associated with hereditary characteristics of purine metabolism and excretion of MUN by the kidneys; - secondary
– the cause of development is some other diseases, nutritional disorders, bad habits, etc.
According to the mechanism of accumulation of MUN, gout is divided into types:
- metabolic
– increased internal production of purines during their normal excretion by the kidneys; - renal
– impaired excretion of uric acid by the kidneys; - mixed
.
By severity:
- mild course
- attacks of gouty arthritis no more than 2 times a year with damage to no more than two joints; single tophi no more than 1 cm in diameter; there are no complications from the kidneys or impaired joint function; - moderate severity
- no more than 5 attacks per year affecting no more than 4 joints with minor changes in cartilage and bone tissue; a large number of small tophi; stones in the kidneys; - severe
– attacks of gouty arthritis more than 5 times a year, multiple large tophi and arthritis; decreased kidney function.
Symptoms of gouty arthritis
The appearance of the first symptoms of gouty arthritis is sometimes preceded by a long-term increase in the concentration of urate in the blood. Therefore, men after 40 years of age, and women after menopause, need to periodically check the content of uric acid salts (UA) in the blood. This is especially important for those who have close relatives suffering from gout. Gouty arthritis develops when the concentration of urate in the blood exceeds 0.4 mmol/l. But with such an indicator, arthritis manifests itself only in a fifth of patients; the rest may not be aware of their risk of developing gout.
Primary signs
The first attack of acute gouty arthritis begins suddenly. Sharp pain appears in the affected joint, the tissue over it swells, and the skin turns red. The pain is very strong. The body temperature may rise and the general condition of the patient may be disrupted.
Severe pain in the affected joint, high body temperature and poor condition of the patient are the first signs of gouty arthritis.
In half of the cases, gouty arthritis begins with damage to one joint. This is usually the first metatarsophalangeal joint of the foot. The knee, elbow, small joints of the hand, etc. may also be affected.
Symptoms of gouty arthritis are especially pronounced at night.
Obvious symptoms
The attack of gouty arthritis lasts from 2 days to 2 – 3 weeks. Then complete remission occurs without any consequences. The next attack usually develops within a year, but sometimes remission lasts several years.
Acute gouty arthritis can recur over a number of years, but gradually its course becomes chronic. Several joints are asymmetrically affected, including the first metatarsophalangeal joint on the lower extremities.
Under the skin on the extensor surface of the joints, as well as in the area of the ears, tophi appear - small superficial nodules or larger subcutaneous nodes with a cartilaginous consistency. They are painless, but can become inflamed during an exacerbation of gouty arthritis. In this case, they become painful and sometimes break through to the surface of the skin in the form of a whitish mass.
Attacks of urolithiasis may occur - urates are deposited on the walls of the urinary tract, as well as in the kidneys, which leads to disruption of their function.
When you need to see a doctor urgently
You should seek medical help if:
- joint pain appeared, accompanied by severe redness and swelling of the tissues; body temperature increased, chills and malaise appeared;
- severe paroxysmal pain appeared in the lower back - an attack of urolithiasis;
- joint pain also bothers you during the interictal period - a sign of the chronic course of gouty arthritis.
How to recognize?
“Gout attacks appear suddenly and mainly in the evening and at night. The main symptom is severe pain, pain increases rapidly over 8-12 hours, severe redness and swelling in the area of the affected joint. There may be chills and a rise in temperature,” says Isabella Andreeva.
You shouldn’t endure it and hope that it will go away on its own. The pain will not subside on its own, says the specialist. And without treatment, there is a high probability that the affected joints will collapse.
Article on the topic
Gout: causes, first signs and treatment
Stages of gouty arthritis
Gouty arthritis occurs in 4 stages:
- Asymptomatic
- Increased levels of MUN in the blood without the presence of crystals and gout attacks. - Asymptomatic
- increased levels of MUN in the blood with the presence of crystals in the synovium and joint fluid, but without signs of gouty arthritis and the presence of tophi. - Intermittent
- deposition of MUN crystals in tissues in combination with attacks of acute gouty arthritis. - Chronic tophi
- the presence of tophi in articular and periarticular tissues in combination with chronic arthritis, destruction of cartilage tissue, impaired joint function and kidney damage.
Any form of arthritis has serious complications, so you should not delay treatment.
See how easily the disease can be cured in 10-12 sessions.
If gouty arthritis is not treated
If you suspect gouty arthritis, you should immediately contact a rheumatologist. The disease requires treatment, both during an attack and in the interictal period. The main reason why the development of gout attacks cannot be brought under control is the refusal of patients to undergo treatment in the inter-attack period, which inevitably leads to:
- hyperuricemia – increased urate levels in the blood;
- resumption of attacks of gouty arthritis;
- transition of acute gouty arthritis to chronic;
- joint destruction and disability;
- severe complications from the kidneys and cardiovascular system.
What to do during exacerbations
If severe joint pain occurs in combination with severe swelling and redness of tissues, increased body temperature, and malaise, you should:
- take any sedative + medicine from the group of non-steroidal anti-inflammatory drugs (NSAIDs) - Diclofenac (oral tablet or rectal suppository), Ibuklin, Nise, etc. Apply ointment or gel from the same group (Voltaren, Pentalgin, etc.) to the skin over the sore joint. ;
- call a doctor at home;
- lie down and take a position that minimizes joint pain.
Drugs for the treatment of exacerbation of gouty arthritis
Criteria for the diagnosis of gout
| Criterion | Joint | Point |
| Clinical | ||
| Joint involvement during a typical gout attack | ankle/tarsus, 1st metatarsophalangeal joint | + 1 point + 2 points |
| Typical acute attack of gout | erythema over the surface of the joint (reported by the patient or recorded by the doctor), inability to touch or apply pressure to the area of the affected joint, significant difficulty walking or inability to perform. | one characteristic “+1 point” two characteristics “+2 points” three characteristics “+3 points” |
| Dynamics of a typical acute attack | The presence of 2 or more signs, regardless of anti-inflammatory therapy:
| one typical episode “+1 point” recurrent typical episodes “+2 points” |
| Clinical signs of tophi | Drained or plaster-like subcutaneous nodule, often vascularized, with typical localization: joints, ears, olecranon bursa, fingertips, tendons. | Presented "+4 points" |
| Laboratory methods | ||
| Uric acid level (determined during the period of time when the patient is not receiving drugs that reduce uric acid levels) | < 4 mg/dl (240 µmol/l) 6- 8- >10 mg/dl (> 600 µmol/l) | “- 4 points” “+2 points” “+3 points” “+4 points” |
| Synovial fluid analysis (polarization microscopy) | Negative result. | "-2 points" |
| Diagnostic Imaging Techniques | ||
| Signs of urate deposits | Ultrasonic “double-loop” phenomenon or signs of urate deposition when using the CT method with two radiation sources. | "+4 points" |
| Signs of gout-related joint damage | Detection of at least 1 erosion during radiography of the hands and/or feet. | "+4 points" |
Example of using diagnostic criteria:
- Attack of arthritis of the first metatarsophalangeal joint - +2 points
- Characteristics of the episode: erythema over the joint, inability to tolerate touch/pressure, great difficulty walking/inability to use the affected joint +3 points
- More than 1 “typical episode of arthritis” – +2 points
- Hyperuricemia (548 µmol/l) – +3 points
Localizations
With gout, gouty arthritis of the joints of the lower extremities most often develops. There may be other localizations, including damage to the joints of the upper extremities. Gout is also characterized by asymmetrical joint lesions.
Gouty arthritis of the lower extremities
During a primary gouty attack, the pathological process in half of the cases involves the 1st metatarsophalangeal joint of the foot. And even if this joint is not the first to be affected, gouty arthritis will still develop in it later. The periarticular tissues swell, the skin turns red. Subsequently, small and large tophi appear on the dorsum of the foot.
Gouty arthritis of the ankle is less common and most cases occur with repeated attacks. The ankle becomes inflamed, swollen and red, and the inflammation spreads to the heel. There is severe pain and the inability to step on the foot.
The knee is often affected, the lesions are asymmetrical, often combined with lesions of the 1st metatarsophalangeal and elbow joints. Severe pain, swelling and redness are initially combined with impaired limb function due to pain, but with prolonged gout, joint deformation and ankylosis (immobility) occur.
Hip gouty arthritis is rare and the redness and swelling are not so noticeable under the thick layer of muscles and ligaments. But the pain can be severe.
Chondroprotectors: what are they, how to choose, how effective are they?
Joint pain at rest
Gouty arthritis of the upper extremities
The small joints of the hand and fingers often become inflamed, and the fingers become like sausages. The pain, inflammation and swelling are very severe. Large tofuses appear on the back of the hand.
The elbow is no less often affected. The lesions are asymmetrical and are often combined with the involvement of small joints of the hand and foot. Small and large tophi appear on the extensor surface of the shoulder and forearm.
Brachial gouty arthritis develops much less frequently, but is painful. Swelling and redness are not expressed, tophi appear on the flexor surface of the shoulder.
Lesions in gouty arthritis of the upper extremities are usually asymmetrical
Tofus lesion of the spine
In the mid-50s of the last century, spinal damage due to gout was first identified. In this case, tophi grow in the soft tissues and joints of the spine with the destruction of their structures.
The lumbar region is most often affected, followed by the cervical region. Pain appears in the back, which is often mistaken for symptoms of osteochondrosis. When the vertebrae are destroyed and the spinal nerves and spinal cord are compressed, neurological symptoms appear. When the cervical spine is affected, this results in paresis and paralysis of the upper limbs, and radicular pain.
When the lumbosacral region is affected, it can be complicated by compression of the final part of the spinal cord - the cauda equina. In this case, the function of the pelvic organs is disrupted - involuntary urination, defecation, and potency disorders occur.
Symptoms of gout in women
Signs of gout in women are completely absent for a long time, although there will already be deviations in the biochemical blood test. Even if there are no signs of gout in women, it may be necessary to prescribe treatment. Therefore, if there are any deviations during a preventive examination, it is better to consult a specialist.
The first signs of gout in women that are visible clinically are pain, swelling, hyperemia and hyperthermia of the joints. Most often, gout manifests itself in the joint of the big toe. The joints of the thumb, ankle, knee and elbow are somewhat less commonly affected. Symptoms of gout in women are characterized by severe pain, severe limitation of mobility, swelling and redness of the joints. The pain is intense, constant, the maximum peak is in the first day. After two or three days, as a rule, in the case of the first attack, the pain goes away on its own.
After a sufficiently long period of “calm”, against the background of full health, a second attack occurs. Over time, the attacks will last longer and longer, and the periods of remission will become shorter. The progression of gout leads to the disappearance of the period between attacks and the disease leads to chronic gouty arthritis.
At the stage of chronicity of the process, the clinical picture already shows specific signs of gout in women: gouty nodules - tophi. Their favorite localization is the ears, fingers, toes, etc. Symptoms of involvement of the urinary system are also added. Nephrolithiasis, pyelonephritis, cystitis, etc. are typical.
With the right treatment, it is possible to achieve long-term remissions, reduce the number of attacks, and make them less intense. Quality treatment can prevent the kidney symptoms of gout in women. The choice of treatment method in the future depends on the presence of signs of gout in women.
Diagnostics
Despite the fact that gouty arthritis has pronounced symptoms, only 10% of patients can be correctly diagnosed during the first attack. In other cases, a diagnosis of other types of arthritis is made. Diagnostic criteria for gout are:
- acute arthritis of the 1st toe;
- the presence of large and small tophi;
- increased levels of uric acid in the blood;
- detection of EOR crystals in joint fluid and tissues.
If at least two criteria are identified, the diagnosis of gouty arthritis is considered reliable.
Laboratory research:
- general blood test
- signs of inflammation; - biochemical blood test
- uric acid content more than 0.32 mmol/l; increased levels of C-reactive protein (a sign of an inflammatory reaction); - general urine analysis
; - examination of synovial fluid using polarization microscopy
- identification of MUN crystals and a large number of leukocytes.
Instrumental studies:
- Ultrasound of joints
- detection of EOR crystals on the surface of cartilage and tophi; - radiography of the joints
- in the early stages there are no changes; later, bone changes are revealed; - computed tomography (CT)
- reveals the presence of changes in the spine.
Treatment of gouty arthritis
The goal of treatment for gouty arthritis is to improve disease outcomes. For this purpose, mainly medicinal treatment methods are used. Non-drug methods are of auxiliary value.
Drug treatment
The main objectives of drug treatment of gouty arthritis are:
- relieving inflammation and pain in acute arthritis;
- preventing attacks of arthritis by reducing uric acid in the blood.
Treatment of an acute attack of arthritis
The following drugs are used to relieve an attack of gouty arthritis:
- Colchicine
is a dry extract of autumn crocus seeds. Available in tablets. Effective in the first 12 hours from the onset of a gout attack. The result of its use is the elimination of swelling and pain. The drug is prescribed in small dosages. The daily dose is divided into several doses. First, most of the daily dose is prescribed, then an hour later - a smaller one. If necessary, the dose can be repeated several times a day. A day after the onset of an attack, minimum dosages cannot be used; they are increased, which contributes to the manifestation of side effects, mainly from the gastrointestinal tract. All dosages are selected by the doctor. - Nonsteroidal anti-inflammatory drugs (NSAIDs)
– relieve inflammation, pain and swelling. These drugs are divided into 2 groups: non-selective and selective. Non-selective or 1st generation NSAIDs suppress the action of biologically active substances - prostaglandins, which support inflammatory processes. But they do not act selectively, also suppressing the action of prostaglandins that protect the gastric mucosa. Therefore, drugs such as Diclofenac, Indomethacin, Ibuprofen have side effects from the gastrointestinal tract (GIT). However, they are suitable for some patients and are often prescribed, both orally and intramuscularly. Selective NSAIDs (Nimesulide, Etoricoxib, Celecoxib) belong to the second generation. They act selectively on pro-inflammatory prostaglandins and have almost no effect on the gastrointestinal tract. The selection of the drug is carried out individually, in accordance with the characteristics of the patient’s body. - Glucocorticoid hormones (GCs)
- quickly relieve inflammation, pain and swelling, but are not suitable for everyone, as they can increase blood pressure, blood sugar and cause exacerbation of peptic ulcer disease. Prescribed in short courses orally (Prednisolone), intramuscularly (Betamethasone), in the form of intra-articular injections. Currently, they are trying to use mainly drugs from the NSAID group and only if there are contraindications for their use, GCs are used. - Canakinumab (trade name Ilaris)
is a monoclonal antibody to interleukin-1beta (IL-1b). IL-1b is a messenger protein molecule (cytokines) responsible for the inflammatory response. Antibodies bind to IL-1b and neutralize its action, which leads to the elimination of inflammation and pain. Canakinumab is used in an individually selected dosage for patients who have contraindications to the use of Colchicine, NSAIDs and GCs.
Drugs for the treatment of gouty arthritis
Decrease in uric acid levels in the blood during the interictal period
Outside of attacks, patients suffering from gouty arthritis are prescribed long-term courses of urate-lowering therapy (UST), which lower the content of uric acid (UA) in the blood serum, preventing the formation of UA crystals in tissues. With a low content of uric acid in the blood, the crystals already present in the tissues gradually dissolve. In the presence of chronic gouty arthritis and tophi, the UA content is reduced to a minimum (below 0.3 mmol/l), which contributes to the accelerated elimination of tophi. In the absence of tophi, an acceptable UA content of 0.36 mmol/l is acceptable.
For urate-lowering therapy for gouty arthritis, the following groups of drugs are used:
- A group of drugs that interfere with the formation of uric acid. They suppress the action of the enzyme involved in the formation of uric acid. When prescribing them, constant monitoring of UA content in blood serum is necessary. These medications include:
- Allopurinol is a drug that has been used to treat gout for more than 50 years. It is prescribed in long courses 2 weeks after the end of a gout attack with small doses, which are gradually increased to the required levels. Sometimes it gives side effects from the kidneys, liver, severe allergic skin reactions. At the initial stage of treatment, it often causes an exacerbation of gouty arthritis, so it is combined with the prescription of NSAIDs.
- Febuxostat (trade names: Adenuric, Azurix) is a more modern drug of this group; it acts selectively on only one enzyme involved in the synthesis of uric acid. It does not inhibit other enzymes, so it has fewer side effects. It works softly and effectively. Doses are selected individually and initially combined with the use of NSAIDs.
- Drugs that enhance the excretion of sUA through the kidneys. Prescribed for intolerance to drugs of the first group or ineffectiveness of their use:
- Probenecid - prescribed in long courses, contraindicated in the presence of kidney stones.
- Enzymes that are absent in humans can reduce the level of sUA in the blood. The enzyme uricase is present in the blood of some mammals and reduces the level of UA in the blood, but it is not present in human blood. The drug rasburicase (recombinant bacterial uricase) was produced using genetic engineering from bacteria, which is used for gouty arthritis if other methods fail to reduce UA in the blood. The disadvantage of the drug is increased allergenicity, so it is used strictly according to indications.
Peguricase is a uricase with polyethylene glycol, which suppresses increased allergenicity. A more modern drug, but it is also used strictly according to indications.
Crunching in joints - when to worry
Intra-articular injections of hyaluronic acid
Non-drug treatment
This type of treatment includes:
- diet;
- control over the course of comorbid (often combined with gout and aggravating its course) diseases;
- physiotherapeutic procedures;
- folk remedies.
Diet for gouty arthritis
Patients suffering from gouty arthritis should not consume foods rich in purine bases. But recent studies have found that only animal products have a negative effect. This is fatty red meat, offal (liver, kidneys, heart, brains), strong meat broths, canned fish in oil, canned meat and pates.
On the contrary, plant foods containing purine bases should not be completely excluded from the diet. These are nuts, peas, beans, lentils, legumes, cocoa and coffee. Dairy-vegetable diets with low-fat dairy products, a variety of vegetables and fruits are very useful. Especially useful are foods rich in vitamin C, which helps eliminate uric acid.
Alcohol should be excluded from the diet: strong spirits and beer, including non-alcoholic beer. Only a small amount of dry wine is allowed. You should not consume sweets, baked goods, and especially sweet carbonated drinks. It is also necessary to stop smoking.
Monitoring the course of comorbid diseases
Gouty arthritis is often combined and aggravated by obesity, diabetes mellitus, cardiovascular and kidney diseases.
Obesity requires constant monitoring of body weight. This includes following a diet with a reduced daily caloric intake, an active lifestyle, and courses of therapeutic exercises.
Diabetes mellitus also requires constant monitoring, laboratory monitoring and supportive treatment.
Frequent concomitant diseases with gouty arthritis are also arterial hypertension (consistently high blood pressure) and coronary heart disease with angina attacks. Currently, there are medications that can stabilize the condition of such patients, but this requires constant monitoring by a cardiologist.
Any renal pathology also complicates the course of gouty arthritis. She also needs to be observed and treated.
Physiotherapeutic procedures
Physiotherapy is an additional treatment for gouty arthritis. During a gouty attack, electrophoresis with glucocorticoid hormones and ultraviolet irradiation of the affected area are prescribed.
Electrophoresis with glucocorticoid hormones and ultraviolet irradiation are used to treat gouty arthritis.
During the period of remission, courses of magnetic and laser therapy are prescribed to improve blood microcirculation and restore damaged joint tissue.
In the absence of gout attacks, sanatorium treatment can be carried out for six months. The Caucasian Mineralnye Vody and Crimea sanatoriums are suitable.
Read about other methods of treating arthritis in this article.
Folk remedies
To relieve inflammation, swelling and pain during a gout attack, traditional medicine recommends compresses with activated carbon. Previously, charcoal was used for this purpose, today it is enough to take 50 tablets of activated carbon, crush, dilute with water to the consistency of homemade sour cream, add a tablespoon of vegetable oil, mix and apply to the inflamed area, apply a napkin, compress paper, cotton wool on top, bandage and leave all night long. Perfectly relieves inflammation, swelling, reduces pain.
Surgery
For large tophi, which often suppurate and compress surrounding tissues, disrupting their function, they are removed.
Surgical operations aimed at restoring joint function are carried out strictly according to indications, in case of destruction of cartilage tissue, proliferation of bone tissue with articular deformation and loss of limb function. If the joint is completely destroyed, it is replaced with an artificial one (endoprosthetics).
Prevention and diet
For patients suffering from gouty arthritis, it is very important to follow a certain diet. The diet includes limiting foods containing purines. These people are shown:
- Cooking meat for a long time;
- Limiting the intake of proteins that contribute to the production of uric acid;
- Exclusion from the menu of coffee and alcoholic drinks, strong tea;
- Reducing the consumption of fatty, spicy foods to a minimum;
- Increasing fluid intake to 2.5-3 liters to remove salts;
- Limit table salt in the diet to 6-8 g per day.
The menu should include berries, vegetables, fruits, sour cream and cottage cheese, vegetable soups, and porridge. There is no ban on flour products, but it is better to avoid baked goods. 2-3 times a week you need to eat fish and boiled meat.
Proper nutrition is one of the measures to prevent gouty arthritis.
Since the development of the disease is promoted by a passion for fatty foods and alcohol, their exclusion already reduces the risk of pathology. You should select the optimal physical activity in accordance with your age and general condition of the body, for example, engage in physical therapy. A sedentary lifestyle is one of the factors contributing to the development of the disease. Excess weight can also be one of the causes of gouty arthritis, so a balanced diet and physical activity are in any case necessary for prevention.
Gouty arthritis can have quite dangerous consequences. To avoid complications and further development of the disease, it is necessary to diagnose it in time and follow the doctor’s recommendations.
Approach to the treatment of gouty arthritis at the Paramita clinic
Our clinic has developed a unique method for treating gouty arthritis. There are two opposing approaches to identifying and treating this disease. First of all, a thorough clinical, laboratory and instrumental examination of the patient is carried out. After establishing the final diagnosis and concomitant diseases, individually selected comprehensive treatment is prescribed, including:
- modern Western techniques, including the use of the latest medications; this allows you to eliminate inflammation and pain during a gout attack and maintain the desired level of uric acid in the blood serum during the inter-attack period;
- traditional oriental techniques that have a regulating effect on the body as a whole and on the site of inflammation; techniques allow you to eliminate pain after the first session; After the course of treatment, the patient feels a surge of strength and complete renewal.
We combine proven techniques of the East and innovative methods of Western medicine.
Read more about our unique method of treating arthritis
A completed course of treatment and properly selected urate-lowering therapy allows the patient to forget about gout attacks for a long time (in most cases until the end of life). You can get more detailed information about treatment at the clinic on our website.
Treatment methods for gout
Treatment for gout is aimed at:
- in case of an acute attack - to relieve it;
- in the remission stage - to normalize uric acid metabolism. Of course, one should strive to eliminate the cause of the disease. However, if the cause of gout is fermentopathy, this is impossible. In this case, treatment will be symptomatic.
- for the treatment of concomitant diseases.
A complete cure for gout is impossible. However, with medical help, it is possible to reduce the frequency of attacks and achieve a significant increase in periods of remission.
General clinical recommendations for patients with gouty arthritis
All patients suffering from gout are advised to:
- follow a diet, lead an active lifestyle;
- follow all doctor's orders, including taking prescribed urate-lowering medications;
- monitor your weight;
- treat concomitant diseases: diabetes, obesity, angina pectoris, high blood pressure, chronic kidney disease.
Prevention of gout
Gout is most often associated with hereditary metabolic characteristics. There are also no factors predisposing to the development of this disease. If you eliminate their influence, then even if you have a family history, you can significantly reduce the risk of developing the disease. To do this, you need to follow the following recommendations:
- do not overeat, reduce the calorie content of your daily diet, do not consume offal, red meat, alcohol, quit smoking;
- get rid of excess weight;
- Take any medications in consultation with your doctor - some of them increase the concentration of uric acid (diuretics, nicotine, acetylsalicylic acid, etc.);
- regularly treat chronic diseases: diabetes, obesity, kidney and cardiovascular diseases;
- men over 40 years old, and women over 50, periodically check the level of uric acid in the blood.
Cardiovascular risk in patients with gout and possible ways to reduce it
Gout is a systemic disease in which monosodium urate crystals are deposited in various tissues and in individuals with hyperuricemia, inflammation develops due to environmental and/or genetic factors [1]. The prevalence of gouty arthritis in the population is quite high and amounts to 5–28 per 1000 men and 1–6 per 1000 women, and the number of new cases per year is 1–3 per 1000 men and 0.2 per 1000 women [2].
Over the past decades, the incidence of gout has increased several times [3]. Despite the detailed description of gout already in the last millennium, this disease remains one of the late diagnosed processes. According to the Clinic of Therapy and Occupational Diseases named after. E. M. Tareev First Moscow State Medical University named after. I.M. Sechenov, of 128 patients with typical gout, in more than half the diagnosis was established extremely late, already with advanced organ damage [4].
Interest in the problem of gout is due not only to the increase in its incidence, but also to the discovered relationships between gout, hyperuricemia and cardiovascular risk [5]. Hyperuricemia plays an important role in the development of arterial hypertension (AH), diabetes mellitus and is an independent predictor of cardiovascular diseases [6].
The pathogenetic mechanisms of increased cardiovascular morbidity and mortality in individuals with hyperuricemia and gout are diverse. Chronic inflammation is one of the factors that triggers and maintains the formation of atherosclerotic plaque in patients with hyperuricemia and gout [7]. Oxidative stress is another important factor contributing to endothelial dysfunction and damage to the vascular wall, underlying the development of heart failure (HF), hypertension, and coronary heart disease (CHD) in patients with rheumatic diseases [8].
According to our data, which coincides with literature data [9], patients with gout belong to the group of patients with a high risk of fatal cardiovascular complications, which is due to existing endothelial dysfunction (increased antithrombogenic activity of the vascular wall, damage to the vascular wall, increased arterial stiffness). Traditional cardiovascular risk factors are more common in patients with gout than in the general population. In our study, 24-hour blood pressure (BP) monitoring revealed hypertension in 91% of patients; the majority of patients with gout (73.7%) had a 24-hour profile with an insufficient degree of nocturnal BP reduction (non-dipper) [10]. In such patients, arterial stiffness and C-reactive protein (CRP) levels are significantly increased (p < 0.05) [11].
In recent years, researchers have also found a relationship between the presence of aortic stenosis and gout, but whether gout is a risk factor or a marker of heart valve damage has yet to be studied [12]. But there is no doubt that damage to the cardiovascular system in gout, along with arthritis, is one of the main clinical manifestations of gout, determining the severity and prognosis of the disease [13]. Cardiovascular morbidity and mortality exceed mortality from renal damage in patients with gout [14].
As an illustration, we present a clinical case of a patient with gout with the development of a fatal cardiovascular complication.
Patient K., 62 years old, resident of the Saratov region, worked as a driver for 38 years. I suffered from gout for 13 years. The disease began in a typical manner, with the onset of acute attacks of arthritis in the joints of the feet, including the first metatarsophalangeal joints and tarsal joints, repeated 3–4 times a year. The attacks were controlled by taking non-steroidal anti-inflammatory drugs (NSAIDs) for several days. After 3 years from the onset of the disease, he consulted a rheumatologist, was diagnosed with gout, and was prescribed allopurinol at a dose of 200 mg/day, but the patient initially demonstrated low adherence to treatment. Allopurinol was taken only for relapse of arthritis, combined with NSAIDs. The frequency of arthritis attacks has been continuously increasing; over the past 8 years there have been no inter-attack periods. From the age of 56, the most rapid growth of subcutaneous tophi was observed, which led to deformation, disfigurement of the limbs, and a pronounced limitation in the range of motion in the joints (hands, feet, knees, ankles, wrists, elbows, shoulders). Since the age of 58, he periodically noted an increase in blood pressure (up to a maximum of 170 and 100 mm Hg), but did not receive constant antihypertensive therapy. Occasionally he took enalapril 20 mg per day.
He was hospitalized in the rheumatology department of the regional clinical hospital in April 2015. On examination: height - 178 cm, body weight - 109 kg, BMI - 34.4 kg/m2.
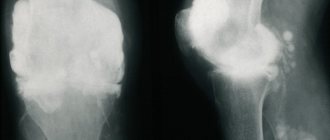
Attention was drawn to subcutaneous formations (tophi) in the area of the ears, the dorsum of the toes, elbows, knee joints, hands, some of large sizes (Fig.); pain, swelling of the joints of the hands and feet, ankle, knee joints; restriction of movements in them. The intensity of joint pain on a visual analogue scale is 80 mm. According to laboratory tests: serum level of uric acid (UA) in the blood - 740 µmol/l (target level 360 µmol/l), creatinine - 98.4 µmol/l, CRP - 10.4 mg/l, ESR - 45 mm/ h. After relief of arthritis, therapy with allopurinol 200 mg/day was resumed. 1 week after the start of therapy, the serum level of uric acid decreased to 504.3 μmol/l. Among the antihypertensive drugs, the patient took losartan 100 mg/day, amlodipine 5 mg/day. Against this background, blood pressure was between 140 and 90 mm Hg. Art. The patient was discharged as an outpatient with recommendations to continue therapy and titrate the dose of allopurinol until the target sUA values were achieved, which the patient did not do. I received therapy irregularly.
In February 2021, for emergency reasons, he was hospitalized in the emergency cardiology department with a clinic for acute coronary syndrome. Despite the full therapy, the patient died 14 hours after admission to the hospital from acute Q-myocardial infarction localized in the posterior wall of the left ventricle.
This clinical observation is not unique, but rather typical. Middle-aged and elderly male patients with metabolic syndrome and tophi gout, without adequate hyperuricemic therapy, extremely often die from cardiovascular complications.
New European guidelines for the management of patients with gout were published in 2021 [15]. One of the reasons for revising previously existing recommendations was the emergence of new drugs. Thus, in recent decades, IL-1 inhibitors have been used for the treatment of acute attacks, along with NSAIDs and colchicine, and non-purine xanthine oxidase inhibitors (KOIs), uricosurics, and uricase preparations have been used as urate-lowering drugs.
In our country, xanthine oxidase inhibitors are represented by two classes of drugs - purine (allopurinol, oxypurinol) and non-purine (febuxostat, topiroxostat).
According to the latest recommendations, allopurinol remains the first-line drug for urate-lowering therapy with preserved renal function. If the target sUA level cannot be achieved with an adequate dose of allopurinol, the drug should be replaced with febuxostat. Febuxostat is indicated for allopurinol intolerance and is the drug of choice for chronic kidney disease [15].
Febuxostat was recently registered in the Russian Federation. The first experience of its use in our country demonstrates the possibility of its use in patients with severe disabling gout [16]. Until recently, the lack of an alternative to allopurinol left virtually no chance of achieving the desired treatment result and preventing the progression of gout, the development of complications associated with gout and hyperuricemia [17].
An important aspect of studying the effectiveness of modern drugs is the effect of anti-gout drugs on cardiovascular risk. Preliminary reports of the use of canakinumab for the prevention of cardiovascular disease in the CANTOS (Canakinumab ANti-inflammatory Thrombosis Outcomes Sudy) study, conducted to test the inflammatory theory of atherosclerosis, results from other studies suggest a beneficial effect of the drug on outcomes associated with atherosclerosis, which is of fundamental importance for patients with gout [18].
Large studies have provided evidence that effective urate-lowering therapy not only reduces the risk of developing new attacks of arthritis and improves the quality of life of patients, but also reduces the risk of cardiovascular disease, kidney damage and mortality [19].
There are conflicting data in the literature regarding the protective effect of COIs on the cardiovascular system [20]. A population-based study in the UK found that taking allopurinol for gout was associated with a 19% reduction in the risk of death [21]. There is evidence of a decrease in the severity of endothelial dysfunction when taking allopurinol in patients with high cardiovascular risk [22].
The literature discusses the possible dose-dependent effect of allopurinol on cardiovascular risk. Thus, in the work of A. Noman et al. (2010), allopurinol in high doses (600 mg/day) in patients with angina pectoris proved to be an effective anti-ischemic agent, and in patients with heart failure, the same high doses of the drug reduced mortality twofold [23]. Whereas in more recent studies there is an indication of the leveling of the positive cardiovascular effect of allopurinol at a dose exceeding 300 mg per day [24], which may be associated with an increase in oxidative stress in conditions of high concentrations of the allopurinol metabolite - oxypurinol [25].
In 2021, the work of M. Bredemeier et al. presents a systematic review and meta-analysis of randomized trials [26], the purpose of which was to analyze the incidence of major adverse cardiovascular events, mortality (overall and cardiovascular) in randomized controlled trials using COIs compared with placebo or no treatment. The analysis included 81 articles (10,684 patients, 6434 patient-years). The results showed that the use of COI did not significantly affect the risk of major adverse cardiovascular events (relative risk (RR) 0.71, 95% confidence interval (CI) 0.46–1.09) and death (RR 0.89, 95% CI 0.59–1.33), but resulted in a reduced risk of total (RR 0.60, 95% CI 0.44–0.82), including major, cardiovascular events (RR 0. 64, 95% CI 0.46–0.89) and hypertension (RR 0.54, 95% CI 0.37–0.80). This also applied to patients with a history of cardiovascular pathology (RR 0.42, 95% CI, 0.23–0.76).
Patients receiving allopurinol had a lower risk of myocardial infarction (RR 0.38, 95% CI 0.17–0.83), hypertension (RR 0.32, 95% CI 0.18–0.58), total number (0.48, 0.31–0.75) and major cardiovascular events (RR 0.56, 95% CI 0.36–0.86). However, this effect was leveled out when the dose of allopurinol was increased above 300 mg/day, which requires further research.
The cardiovascular effects of non-purine xanthine oxidase inhibitors are less studied. As a result of selective inhibition of two forms of xanthine oxidase (oxidized and reduced), when taking febuxostat, the concentration of UA in the patient’s blood serum decreases.
The effect of febuxostat on insulin resistance (IR) and the expression of high-sensitivity CRP (hs-CRP), which is important both in the treatment of gout and in reducing cardiovascular risk, has been described [27]. In a study by J. Meng et al. 42 patients with gout and 20 control subjects, matched by gender and age, were included. Fasting insulin and blood glucose levels and hs-CRP were determined. IR was assessed using the HOMA-IR index. Patients with gout had higher levels of uric acid, insulin, HOMA-III index and hs-CRP levels than the control group (p < 0.05). After 4, 12 and 24 weeks of treatment with febuxostat, the concentrations of sUA and hs-CRP decreased significantly (p < 0.05). Insulin levels and the HOMA-IR index decreased slightly after 4 weeks of therapy and decreased significantly after 12 and 24 weeks of treatment. Thus, febuxostat can effectively control serum UA levels and improve insulin sensitivity in patients with gout.
Non-purine xanthine oxidase inhibitors have antioxidant properties by reducing the number of metabolites of purine metabolism and improving endothelial function [28]. There are studies suggesting benefits of febuxostat over allopurinol in reversing endothelial dysfunction [28] and even improved scores in patients treated with febuxostat compared with allopurinol [29]. At the same time, according to the instructions for medical use of febuxostat, the use of the drug is not recommended in patients with coronary artery disease or congestive heart failure, since, according to the results of long-term large-scale studies, in the group of patients receiving febuxostat, compared with the group of patients receiving allopurinol, there was an increase number of cardiovascular system disorders [30]. There were numerically more cardiovascular events in the febuxostat group for the composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. However, these differences with the allopurinol groups did not reach statistical significance and were not replicated in the CONFIRMS study [31].
Randomized clinical trials are currently ongoing to evaluate the effect of febuxostat on cardiovascular risk in patients with gout [32].
Thus, patients with gout have an increased cardiovascular risk. Adequate urate-lowering therapy can not only normalize blood uric acid levels, but also improve the life prognosis of patients with gout by reducing the risk of developing cardiovascular diseases. In the clinical observation we presented, the lack of adequate urate-lowering therapy was one of the factors in the development of an unfavorable outcome. Further research is required to clarify the mechanisms and features of the effect of various xanthine oxidase inhibitors on cardiovascular risk.
Literature
- Rheumatology. National leadership / Ed. E. L. Nasonova. M.: GEOTAR-Media, 2008. 372 p.
- Barskova V. G., Nasonova V. A. Gout: clinical course, diagnosis, treatment. Quality of life // Medicine. 2003. No. 3. P. 49–53.
- Arromdee E., Michet CJ, Crowson CS Epidemiology of gout: is the incidence rising? // J. Rheumatol. 2012; 29: R. 2403–2406.
- Mukhin N. A. Gout: faces of the disease // Modern rheumatology. 2007. No. 1. P. 5–9.
- Eliseev M. S., Denisov I. S., Markelova E. I. et al. Independent risk factors for the development of severe cardiovascular complications in men with gout: results of a 7-year prospective observation // Therapeutic archive. 2021. No. 89 (5). pp. 10–19.
- Feig DI, Kang DH, Johnson RJ Uric acid and cardiovascular risk // N. Engl. J. Med. 2008; 359(17): P. 1811–1821.
- Perez-Ruiz F., Becker MA Inflammation: a possible mechanism for a causative role of hyperuricemia/gout in cardiovascular disease // Curr. Med. Res. Opin. 2015; 31. Suppl 2: 9–14.
- Richette P., Perez-Ruiz F., Doherty M. et al. Improving cardiovascular and renal outcomes in gout: what should we target? //Nat. Rev. Rheumatol. 2014; 10. P. 654–661.
- Zhu Y., Pandya B., Choi H. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008 // Am. J. Med. 2012: 125. P. 679–687.
- Rebrov A.P., Magdeeva N.A. Use of amlodipine for arterial hypertension in patients with gout // Saratov Journal of Medical Scientific Research. 2008. No. 1 (19). pp. 72–75.
- Rebrov A.P., Magdeeva N.A. Features of endothelial dysfunction in patients with gout and its changes during therapy // Saratov Journal of Medical Scientific Research. 2008. No. 3 (21). pp. 59–62.
- Chang K., Yokose C., Tenner C. et al. Association between gout and aortic stenosis // Am. J. Med. 201 7; 130 (2): 230.e1–230.e8.
- Eliseev M. S., Denisov I. S., Markelova E. I. et al. Independent risk factors for the development of severe cardiovascular complications in men with gout: results of a 7-year prospective study // Therapeutic archive. 2021. No. 89 (5). pp. 10–19.
- Krishnan E., Baker JF, Furst DE et al. Gout and the risk of acute myocardial infarction // Arthritis Rheum. 2006 Aug; 54 (8): pp. 2688–2696.
- Eliseev M. S. Updated EULAR recommendations for the treatment of gout. Comments on some positions // Scientific and practical rheumatology. 2021. No. 55 (6). pp. 600–609.
- Eliseev M. S., Shayakhmetova R. U. Experience of using febuxostat in a patient with severe disabling gout // Modern Rheumatology. 2021. No. 11 (3). pp. 81–84.
- Eliseev M. S., Barskova V. G., Denisov I. S. Dynamics of clinical manifestations of gout in men (data from a 7-year retrospective observation) // Therapeutic archive. 2015. No. 87 (5). pp. 10–15.
- Eliseev M. S., Zhelyabina O. V., Markelova E. I. et al. Assessment of cardiovascular risk when using an interleukin 1 inhibitor in patients with severe tophi gout // Modern rheumatology. 2016. No. 10 (1). pp. 7–14.
- Goicoechea M., de Vinuesa S.G., Verdalles U. et al. Effect of Allopurinol in Chronic Kidney Disease Progression and Cardiovascular Risk // Clin. J. Am. Soc. Nephrol. 2010; 5 (8): pp. 1388–1393.
- Higgins P., Dawson J., Lees KR et al. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis // Cardiovasc. Ther. 2012; 30. P. 217–226.
- Dubreuil M., Zhu Y., Zhang Y. et al. Allopurinol initiation and allcause mortality in the general population // Ann. Rheum. Dis. July 2015; 74 (7). P. 1368–1372.
- Xin W., Mi S., Lin Z. Allopurinol therapy improves vascular endothelial function in subjects at risk for cardiovascular diseases: a meta-analysis of randomized controlled trials // Cardiovasc. Ther. 2016; 34. P. 441–449.
- Noman A., Ang D.S., Ogston S. et al. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomized, placebo controlled crossover trial // Lancet. 2010; 375 (9732). P. 2161–2167.
- Givertz MM, Anstrom KJ, Redfield MM, Deswal A. Effects of Xanthine Oxidase inhibition in Hyperuricemic heart failure patients: the Xanthine Oxidase inhibition for Hyperuricemic heart failure patients (EXACT-HF) study // Circulation. 2015; 131. R. 1763–1771.
- Stamp LK, Turner R., Khalilova IS, Zhang M. et al. Myeloperoxidase and oxidation of uric acid in gout: implications for the clinical consequences of hyperuricaemia // Rheumatology (Oxford). 2014; 53. R. 1958–1965.
- Bredemeier M., Lopes LM, Eisenreich MA et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials // BMC Cardiovasc. Discord. 2021, Feb 7; 18(1):24.
- Meng J., Li Y., Yuan X., Lu Y. Effects of febuxostat on insulin resistance and expression of high-sensitivity C-reactive protein in patients with primary gout // Rheumatol. Int. Feb 2021; 37(2). P. 299–303.
- Tsuruta Y., Kikuchi K., Tsuruta Y. et al. Febuxostat improves endothelial function in hemodialysis patients with hyperuricemia: a randomized controlled study // Hemodial. Int. 2015; 19. P. 514–520.
- Tausche AK, Christoph M., Forkmann M. et al. As compared to allopurinol, urate-lowering therapy with febuxostat has superior effects on oxidative stress and pulse wave velocity in patients with severe chronic tophaceous gout // Rheumatol Int. 2014; 34. P. 101–119.
- Schumacher HR Jr., Becker MA, Wortmann RL et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, doubleblind, parallel-group trial // Arthritis Rheum. 2008; 59 (11). P. 1540–1548.
- Becker MA, Schumacher HR, Espinoza LR et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial // Arthritis Res. 2010, 12 (2): R63.
- MacDonald TM, Ford I., Nuki G. et al. Protocol of the Febuxostat versus Allopurinol streamlined trial (FAST): a large prospective, randomized, open, blinded endpoint study comparing the cardiovascular safety of allopurinol and febuxostat in the management of symptomatic hyperuricaemia // BMJ Open. 2014; 4:e005354.
N. A. Magdeeva, Candidate of Medical Sciences I. A. Romanova, Candidate of Medical Sciences N. M. Nikitina1, Doctor of Medical Sciences, Professor
FSBEI HE SSMU im. V. I. Razumovsky Ministry of Health of the Russian Federation, Saratov
1 Contact information
Cardiovascular risk in patients with gout and possible ways to reduce it / N. A. Magdeeva, I. A. Romanova, N. M. Nikitina For citation: Attending physician No. 9/2018; Page numbers in the issue: 82-85 Tags: gouty arthritis, cardiovascular complications, risk group, hypertension
Frequently asked questions about the disease
Is it possible to get disability?
For chronic tophi gouty arthritis with impaired joint function.
Which doctor treats you?
Rheumatologist.
What prognosis do doctors usually give?
With proper systematic treatment under the supervision of a physician, the prognosis is favorable.
Gouty arthritis requires constant monitoring by a rheumatologist, urate-lowering therapy, diet and all doctor’s recommendations. If treated correctly, you can forget about gout attacks forever. Doctors at the Paramita clinic have extensive experience in treating gout. Contact us!
Literature:
- Fedorova A. A., Barskova V. G., Yakunina I. A., Nasonova V. A. Short-term use of glucocorticoids in patients with prolonged and chronic gouty arthritis. Part III. Frequency of development of adverse reactions // Scientific and practical rheumatology. 2009; No. 2. pp. 38–42.
- Eliseev M. S. Gout. In the book: Russian clinical guidelines. Rheumatology / Ed. E. L. Nasonova. M.: GEOTAR-Media, 2021. pp. 372–385.
- Rainer TH, Cheng CH, Janssens HJ, Man CY, Tam LS, Choi YF Oral prednisolone in the treatment of acute gout: a pragmatic, multicenter, double-blind, randomized trial // Ann Intern Med. 2016; 164(7):464–471.
- Reinders M., van Roon E., Jansen T., Delsing J., Griep E., Hoekstra M. et al. Efficacy and tolerability of urate-lowering drugs in gout: a randomized controlled trial of benzbromarone versus probenecid after failure of allopurinol // Ann Rheum Dis. 2009; 68:51–56.
Themes
Arthritis, Joints, Pain, Treatment without surgery Date of publication: 01/25/2021 Date of update: 02/02/2021
Reader rating
Rating: 4.54 / 5 (13)
Which doctor treats gout?
For treatment of gout, you should consult a rheumatologist. The help of an orthopedic doctor may be required (for example, if surgery is necessary).
Treatment of an acute attack
First of all, the patient needs rest. The inflamed joint should be immobilized. It is recommended to apply cold. You need to drink plenty of alkaline fluids - up to 3 liters per day. It is important to follow a diet: foods high in purine bases should be excluded. Painkillers (NSAIDs) and glucocorticoids are used as prescribed by a doctor. Local treatment is carried out using ointments and gels that have anti-inflammatory and analgesic effects.
When the patient feels better, physical therapy is included in the treatment. Electrophoresis, UV irradiation, and UHF are used.
Treatment of gout in remission
Treatment in remission includes:
- lifestyle changes (primarily giving up alcohol);
- diet (exclude foods that contain a lot of purines - fish, mushrooms, legumes). The diet must be prescribed by a doctor;
- drug therapy (anti-gout drugs);
- local treatment - applications of medications, physiotherapy, massage, medicinal baths (in sanatoriums);
- surgical treatment: removal of tophi that are not amenable to conservative treatment, surgical restoration of affected joints.
Make an appointment Do not self-medicate. Contact our specialists who will correctly diagnose and prescribe treatment.
Rate how useful the material was
thank you for rating