The human body consists of a huge number of cells. They, in turn, are combined into larger units - tissues and organs. Organ systems are built from the latter, and from them the organism is formed. And at all levels, various interactions and complex biochemical reactions are constantly taking place. In order for all this to develop harmoniously from a fertilized egg and function smoothly, a special work scheme is required for all levels. Its role in the body is performed by organized genetic information.
It is the hereditary material that is entrusted with the task of preserving data about the general appearance of a person, the structural features of individual organs, the principles of regulation with the help of hormones, and even how proteins should be assembled. All data is recorded as a sequence of structural units - nucleotides. They, like the letters of the alphabet, form word groups that perform specific functions. Each group is called a gene. Together they form the DNA molecule. If you look at a higher level, you will notice that all the hereditary material in the form of chains of deoxyribonucleic acid is concentrated in special formations called chromosomes.
A person normally has 23 pairs of them. In each pair, information is stored in two copies. This is necessary so that when genetic information is transferred, each daughter cell receives its own copy. One pair of chromosomes is different from the rest and is responsible for determining sex. If it has two identical large chromosomes (XX variant), then the organism is female. If there is one large and one small chromosome, then we are talking about the male sex (XY variant). These two different chromosomes are called sex chromosomes. The remaining 22 pairs are found in all organisms, regardless of gender. They are called autosomes.
Anomalies of genetic material
Hereditary material consists of a huge number of nucleotides that form genes. Moreover, in each gene the nucleotide sequence is strictly defined, since it must code for a specific protein. In addition, the genes themselves, when forming chromosomes, are also arranged in a fixed order. Thanks to the preservation of this order, the body can function, and scientists can quickly and accurately indicate to each other which gene we are talking about.
Ideally, the system works without the slightest glitch, and genetic information is always transmitted unchanged. However, in practice, a large number of structural units and constant exposure to various factors (for example, ionizing radiation) leads to the fact that various anomalies arise from time to time. In particular, individual sections of the DNA sequence can be copied to a new location. In this case, they talk about duplication. If, instead of creating a new copy, part of the original chain was moved, then the modification is called translocation. In addition, sometimes part of the sequence is simply lost, removed from the genetic material. In this case, the change is called a deletion.
Since the interactions in the body have been honed over many millennia of evolutionary development, the result is a very coherent system. And anomalies, even the smallest ones, can cause an imbalance. In this case, one or another disorder develops in the body. If the cause is at the level of genes, then they speak of gene diseases. If an extra copy of a chromosome is lost or, on the contrary, gained, such disorders are called chromosomal diseases.
Symptoms and signs
The clinical picture of the disease is very diverse and may differ in each specific case. In particular, the child exhibits physical and mental abnormalities , some signs are characteristic of all cases, others are much less common.
| Physical abnormalities | Psychical deviations |
|
|
Clinical manifestations of the disease are also usually divided depending on their prevalence. Thus, there are symptoms that occur in all children suffering from this disease, signs that occur in most cases (but not always) and more rare manifestations.
| Characteristic symptoms | Common signs | More rare manifestations |
|
|
|
The clinical manifestations of the disease change as the child grows older . Thus, in adolescent children there is a delay in puberty (while childbearing function is not impaired in the future), and when the child becomes an adult, he looks much younger than his peers.
Often, older children experience other manifestations of the disease , such as difficulty urinating, complex motor skills of the hands, problems with communication (the teenager understands spoken language, but does not see the need to respond to the interlocutor).
What is the life expectancy of children with Williams syndrome? Find out the answer right now.
What is microdeletion syndrome?
The most minor changes (aka mutations) are called point changes. Their appearance affects a few units of genes. In some cases, the disorder relates to just one gene. However, if it provided the production of an important protein, the consequences for the entire body could be very serious. Such pathological changes belong to the group of microdeletion syndromes.
Each such disease is caused by a small change in genetic material that occurs in a strictly defined location. The exact mechanism of occurrence of such disorders has not been established to date, which does not prevent scientists from studying their effects on the body.
Thus, it was found that the development of the syndrome in this case can occur in several different ways. In particular, a number of diseases are characterized by the participation of oncogenes. In other cases, the effect of the deletion itself is superimposed by the effect of chromosomal imprinting and possible uniparental disomies.
The incidence of most microdeletion syndromes is extremely low: about 1 case per 50-100 thousand newborns. A set of clinical signs is usually expressed clearly. In order to make a diagnosis, only a combination of symptoms is enough. However, with this approach it is impossible to accurately predict the health of the offspring, so often, along with checking the usual signs, a molecular genetic diagnosis of the proband and his relatives (usually parents, in some cases also requires analysis of the genotype of brothers, sisters, aunts, uncles, and so on) is carried out.
Pathological manifestations vary greatly. In particular, their manifestation is determined by how much of the genetic material was lost as a result of the deletion. In addition, in some cases, the role from which parent the mutation was received (the influence of chromosomal imprinting) plays a role. A good illustration of the latter situation is the pair of Prader-Willi and Angelman syndromes. They are both caused by the presence of a deletion on chromosome 15. However, due to the different mechanism of action when transmitted from different parents, the clinical picture of these diseases differs significantly.
History[edit]
"Boy with a Doll" or "Child with a Drawing", circa 1520, Giovanni Francesco Caroto; portrait of a possible case of Angelman syndrome. [35]
Harry Angelman, a pediatrician practicing in Warrington, England, first reported three children with the condition in 1965. [10] Angelman later described his choice of the title "Child Puppets" to describe these incidents as being related to an oil painting he saw while on vacation in Italy:
The history of medicine is full of interesting stories about the discovery of diseases. The saga of Angelman syndrome is one such story. Quite by chance, almost thirty years ago (eg around 1964), three disabled children were admitted at different times to my children's ward in England. They had different ailments, and although at first glance they seemed to be suffering from different diseases, I felt that their illness had a common cause. The diagnosis was purely clinical because, despite technical research, which has become more sophisticated today, I was unable to establish scientific proof that all three children had the same defect. Because of this, I hesitated to write about them in medical journals. However, while on vacation in Italy, I happened to see an oil painting in the Castelvecchio Museum in Verona called... Boy with a Doll. The boy's laughing face and the fact that my patients were moving convulsively gave me the idea of writing an article about three children called "Child Puppets." Not all parents liked this name, but it served as a means of uniting the three young patients into a single group. The name was later changed to Angelman syndrome. This article was published in 1965 and, after some initial interest, remained almost forgotten until the early eighties.
- Angelman, quoted by Charles Williams [36]
Case reports from the United States first began appearing in the medical literature in the early 1980s. [37] [38] In 1987, it was first noted that approximately half of children with AS are missing a small piece of chromosome 15 ( partial deletion of chromosome 15q
). [39]
Positive effects of some deletions on viability
Small deletion changes can have a significant impact on the survival of the organism. For example, the loss of the gene encoding the CCR5-δ32 protein causes immunity to the human immunodeficiency virus. Scientists suggest that this mutation first appeared about 2.5 thousand years ago and over time spread across Europe.
The current distribution is uneven. According to statistics, about 10% of residents of European countries are resistant to HIV. However, in the Scandinavian countries this figure reaches 14-15 percent. Russians and Finns demonstrate a 16 percent level of resistance. For Sardinia, however, the frequency is a modest 4 percent.
A number of scientists have hypothesized that such a spread is determined by the bubonic plague epidemics that took place in the medieval period. It is likely that a mutation in the gene causes increased resistance to this disease. Therefore, in the countries where the Black Death took place, more people with this genotype survived.
The effect of deletions on the ability to fertilize
Deletions that occur on normal chromosomes (autosomes) can in some cases be compensated for by a normal copy of the gene. However, when it comes to sex chromosomes, especially the Y chromosome, the situation changes.
First of all, it should be noted that the genes localized on it do not have a second copy. With a normal number of chromosomes in the set, the Y chromosome turns out to be extremely vulnerable. Combined with a small number of genes, this leads to serious consequences for each change. Of particular interest are mutations affecting the AZF locus and the SRY gene.
Abnormalities of the SRY gene (Sex-determining Region Y)
The SRY gene, as its name suggests, is responsible for a very important function. It is its presence in the chromosome set that triggers the process of formation of the body according to the male phenotype and stimulates the development of the corresponding genital organs.
The presence of even a small deletion in this gene disrupts the mechanism of sex differentiation. As a result, with a normal karyotype of 46XY, the embryo begins to develop as a female organism. For this reason, the SRY gene accounts for the largest number of mutations associated with underdeveloped gonads. In addition, changes in this gene cause sex inversion.
Abnormalities of the AZF locus
There is also a special region on the Y chromosome that controls the process of sperm production. This is what determines how effective spermatogenesis will be. In addition, the condition of this area affects the properties of sperm, such as the total number in the ejaculate, the ability to move, the presence of structural changes and the ability to fertilize. Only in the presence of well-formed motile sperm can male genetic material be delivered to the egg. In other words, a man’s ability to have children depends on the state of this small section of genetic code.
If there are abnormalities in the AZF locus, the process of sperm production is disrupted. As a result, azoospermia and oligozoospermia may develop. With these pathologies, the ejaculate either does not contain sperm at all, or their number is greatly reduced.
The AZF locus itself is divided into three parts with specific tasks. They are named by adding a suffix: AZFa, AZFb and AZFc. The resulting deletion can remove a fragment of a separate part, or its entirety, or capture two regions at once. With complete removal of the AZF, severe damage to spermatogenesis develops. Partial deletions can manifest themselves in different ways. At the same time, the degree of manifestation of pathology is influenced by the size of the lost fragment and its location in the locus. Therefore, for prognostic purposes, it is extremely important to know where the deletion occurred. In addition, this information can be used for proper family planning and in vitro fertilization.
If the deletion removed the entire locus or any of the regions with a/b indices, then the man cannot produce viable sperm. If the deletion can be described by the formula AZFb/AZFb+, then azoospermia develops due to severe disturbances in the process of sperm formation.
Deletions of the AZFc region lead to the manifestation of pathological symptoms of varying severity. It is also possible to develop oligospermia, which in principle allows conception. In 50-70 percent of the total number of such cases, it is possible to obtain sperm for further use in artificial insemination methods. Partial deletion of the AZFc region can be expressed in the form of various disorders from normozoospermia to azoospermia.
All deletions in the AZF locus that cause one or another pathological situation are the causes of male infertility. Determination of the mutation is possible by histological analysis of seminal fluid. In this case, it is necessary to stop sperm maturation or detect immature sperm. To obtain accurate data on deletions in the AZF locus, PCR of 6 markers is used, which relate to individual sections of the locus.
Causes and provoking factors
The main cause of the development of the disease is considered to be a change or absence of one of the fragments of chromosome 15 . The risk of developing pathology in a child increases if his close relatives (parents) have the following genetic abnormalities:
- The presence of extra chromosomes (one or more).
- Chromosomal inversion, when one of the sections of the chromosome is turned upside down, while part of its fragment is lost, and the genes are located in the opposite order.
- Rearrangement of the Y chromosome, when 2 sections of it change places, while some fragments of the mutated chromosome may be missing.
- Loss of one or more chromosomal fragments.
- Attachment of a fragment of one chromosome to another.
- The presence of extra genes resulting from the replication of one or more chromosomes.
- The ring structure of a chromosome is when its ends are connected to each other, forming a ring.
Angelman syndrome
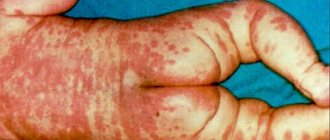
With Angelman syndrome, a characteristic set of pathological changes develops. In particular, there is a delay in psychological development, accompanied by problems with sleep, frequent chaotic movements (mostly with the hands), constant smiling and laughter.
Pathology develops in the absence of certain genes located on chromosome 15. In this case, a mandatory condition is the transfer of a mutant copy of the gene from the mother. If the damaged chromosome is inherited from the father, Prader-Willi syndrome will develop. The karyotype is usually normal (46XX and 46XY for girls and boys, respectively). Various independent studies indicate that the disease is associated with the UBE3A gene, which normally produces an enzyme component in a complex protein degradation system.
The frequency of occurrence of the syndrome is approximately 1 case per 10-20 thousand newborns (indicators differ among different scientists).
The characteristic features of patients with Angelman syndrome are the following:
· nutritional problems that begin during breastfeeding, as children do not gain weight well (prevalence of the symptom is about 75 percent);
· inhibited development of general motor skills, that is, children begin to sit and walk later than others;
· All children are characterized by speech development disorders;
· patients usually understand more than they are able to express using a limited vocabulary;
· often the disease is accompanied by attention deficit and hyperactivity;
· problems with learning in a regular school;
· 80% of patients develop epilepsy, accompanied by disturbances noticeable on electroencephalography; Scientists believe that epilepsy is a secondary (symptomatic) disease.
· performing unusual movements, which include random chaotic movements of the limbs, small tremors;
· occurrence of attacks of laughter in the absence of visible reasons;
· characteristic walking on stiff legs, which gave rise to comparisons with puppets;
· a head reduced in comparison with the average size, often with a flattened occiput;
· in some cases, there are peculiar memorable facial features - a wide mouth with sparsely spaced teeth, a protruding chin with a protruding tongue;
· various sleep disorders;
· in approximately 40 percent of cases, strabismus develops;
· about 10% of patients also suffer from spinal curvature;
· high temperatures are perceived with increased sensitivity;
· the greatest comfort is usually achieved in water (for example, in a bath)
As a rule, the syndrome is determined using molecular genetic diagnostic methods using chromosome 15. An indication for testing for a newborn is decreased muscle tone (hypotonia), a noticeable delay in the development of speech and fine motor skills. In addition, the disease may be indicated by small tremors, jerky, erratic movements, and walking on stiff legs.
The analysis can be carried out through fluorescence in situ hybridization, DNA methylation in the 15q11-q13 region. You can also check for mutations in the imprinting center and in the UBE3A gene.
Since the disease is caused by a genetic disorder, there is no adequate and effective treatment for it. Carrying out therapeutic measures, such as massage for patients with hypotension, can improve the quality of life.
Child care and disease prognosis
The disease is usually detected when the child is between the ages of three and seven years. Symptoms are clearly expressed when reaching this age, not earlier. Since the symptoms are nonspecific, it is difficult to diagnose the syndrome in question in an infant. It is important to note that the disease is relatively rare. Therefore, the doctor, most likely, will not be able to guess that he is dealing with Angelman syndrome.
In Europe there is more data about the pathology in question, so their diagnosis is better and faster. The diagnosis must be made by a geneticist who prescribes a genetic analysis, on the basis of which conclusions are drawn.
The prognosis of the disease correlates with the degree of damage to the fifteenth chromosome. Some patients behave normally in everyday life, their communication is also almost normal. But in some cases the child cannot walk, his speech is limited. People with Angelman syndrome live 20-50 years. The quality of life largely depends on the severity of the syndrome, as mentioned above.
The understanding and attention of his family is important for the life of the patient. They must understand the child and give him all the love they can so that his mental development is complete. Some children have to be sent to a boarding school or specialized school. There they are trained in special programs and learn to live in society.
Prader-Willi syndrome
This disease is determined by the same genetic mutation as for Angelman syndrome. The difference is that in this case, a violation of the hereditary material occurs on the father’s side. The karyotype is normal (46XX or 46XY). The prevalence (1 case per 12-15 thousand newborns) approximately coincides with the prevalence of Angelman syndrome.
Characteristic signs of Prader-Willi syndrome are the following symptoms:
· in the prenatal period, low fetal mobility;
· incorrect position of the fetus is common;
Possible hip dysplasia;
· by the age of two, a tendency to eat a lot (more than normal) may appear, which leads to obesity;
· low muscle tone (hypotonia), combined with impaired coordination of movements;
· feet and hands are usually small, and short stature is also characteristic;
· formation of strabismus and scoliosis;
· note increased drowsiness;
· bone density is at a lower level than in healthy people;
· saliva is thick, teeth are usually in poor condition;
· insufficient function of the gonads, ultimately causing infertility;
· later puberty compared to peers;
· patients learn to speak later and lag behind in mental development;
· external signs include a pronounced bridge of the nose, a narrow and high forehead, almond-shaped eyes, and narrow lips.
In most cases, a person with the mutation will have one to five signs of the disease.
Diagnosis of the disease is carried out by molecular genetic testing, for which children with reduced muscle tone are referred. Often, instead of a correct diagnosis, the more common “Down syndrome” is determined. An experienced geneticist who often encounters manifestations of Prader-Willi syndrome is able to diagnose it based on a set of external signs.
Links[edit]
- ^ abc Angelman syndrome, taken from the Oxford English Dictionary Lexico.com
- ^ abcd Angelman Syndrome in the Merriam-Webster.com Medical Dictionary
- Winter, Robin M.; Baraitser, Michael (2013). Multiple congenital anomalies: a diagnostic compendium. Springer. paragraph 34. ISBN 9781489931092. Archived November 5, 2021.
- Angelman syndrome in the McGraw-Hill Dictionary of Scientific and Engineering Terms
- ^ ab Angelman syndrome in the American Heritage Medical Dictionary
- Angelman syndrome in comprehensive developmental neurobiology: neural circuit development and function in healthy and diseased brains: Chapter 32.
- ^ abcdefghijklmnop Link, Genetics Home (May 2015). "Angelman Syndrome". A Home Guide to Genetics
. Archived from the original on August 27, 2021. Retrieved April 28, 2017. - ^ abcdefghijk "Angelman Syndrome - NORD (National Organization for Rare Disorders)". NORD (National Organization for Rare Diseases)
. 2015. Archived November 13, 2021. Retrieved April 28, 2017. - ^ab "Common Misdiagnoses | FAST" . FAST (Foundation for Angelman Syndrome Therapy)
. Retrieved October 10, 2021. - ^ab Angelman, Harry (1965). "Children Are Puppets: A Report of Three Cases." Dev Med Child Neurol
.
7
(6):681–688. DOI: 10.1111/j.1469-8749.1965.tb07844.x, S2CID 53730099. - Wilson, Golder N.; Cooley, W. Carl (2000). Preventive management of children with congenital anomalies and syndromes. Cambridge University Press. item 193. ISBN. 9780521776738. Archived November 5, 2021.
- Kumar, Vinay; Abbas, Abul K.; Aster, John S. (2013). Robbins Basic Pathology. Elsevier Health Sciences. p. 244. ISBN 978-1437717815. Archived from the original on November 5, 2021.
- ↑ Facts about Angelman syndrome (PDF).
Archived May 27, 2013, at the Wayback Machine . Anonymous. Website of the Angelman Syndrome Foundation (USA). Retrieved September 29, 2012. - Weeber E, Levenson J, Sweatt J (2002). "Molecular genetics of human cognition". Molecular Interventions
.
2
(6): 376-91, 339. DOI: 10.1124/mi.2.6.376. PMID 14993414. - Wang, Yiyang et al. (2017). "Identification of E6AP ubiquitination targets by orthogonal ubiquitin transfer". Nature Communications
.
8
(1): 2232. Bibcode: 2017NatCo...8.2232W. DOI: 10.1038/s41467-017-01974-7. PMC 5738348. PMID 29263404. - Mabb, A. M.; Judson, M.C.; Zylka, M.J.; Philpot, B. D. (May 2011). "Angelman syndrome: understanding genomic imprinting and neurodevelopmental phenotypes". Trends Neurosci
.
34
(6):293–303. DOI: 10.1016/j.tins.2011.04.001. PMC 3116240. PMID 21592595. - Cassidy, S. B.; Dykens, E (2000). "Prader-Willi and Angelman syndromes: nursing imprinted disorders." Am J Med Genet
.
97
(2): 136–146. DOI: 10.1002/1096-8628(200022)97:2 <136::help-ajmg5>3.0.co; 2-V. PMID 11180221. - White HE, Durston VJ, Harvey JF, Cross NC (2006). "Quantitative analysis of SNRPN gene methylation (SRPNN correction) by pyrosequencing as a diagnostic test for Prader–Willi syndrome and Angelman syndrome". Clin. Chem
.
52
(6):1005–13. DOI: 10.1373/clinchem.2005.065086. PMID 16574761. - ^ abc Williams C (2005) "Neurological aspects of Angelman syndrome" Brain and Development 27:88–94
- Laan, Laura AEM; Vienna, Alla A. (2005). “Angelman syndrome: is there a characteristic EEG?” Brain and Development
.
27
(2): 80–87. DOI: 10.1016/j.braindev.2003.09.013. ISSN 0387-7604. PMID 15668045. Kirillovich 5912. - Deng, B., Angelman syndrome: current understanding and research perspectives. Epilepsy, 2009. 50 (11): p. 2331–2339.
- Sidorov, Mikhail S.; Deck, Gina M.; Dolatshahi, Marjan; Thibert, Ronald L.; Bird, Lynne M.; Chu, Catherine J.; Philpot, Benjamin D. (May 8, 2021). "Delta rhythmicity is a reliable EEG biomarker in Angelman syndrome: a parallel mouse and human analysis". Journal of Neurodevelopmental Disorders
.
9
: 17. DOI: 10.1186/s11689-017-9195-8. ISSN 1866-1955. PMC 5422949. PMID 28503211. - ^ abc Frohlich, Joel, Megan Miller, Lynn M. Bird, Pilar Garces, Hannah Purell, Marius K. Hoehner, Benjamin D. Philpot, et al. "The electrophysiological phenotype of Angelman syndrome differs between genotypes." Biological Psychiatry
(2019). - Jana N. R. (2012). "Understanding the pathogenesis of Angelman syndrome through animal models". Neuroplasticity
.
2012
: 1–10. DOI: 10.1155/2012/710943. PMC 3399338. PMID 22830052. - Williams CA, Angelman H, Clayton-Smith J et al (1995). “Angelman syndrome: consensus on diagnostic criteria. Angelman Syndrome Foundation." I am. J. Med. Genet
.
56
(2): 237–8. DOI: 10.1002/ajmg.1320560224. PMID 7625452. - Williams CA, Beaudet AL, Clayton-Smith J et al (2006). "Angelman syndrome 2005: updated consensus on diagnostic criteria". I am. J. Med. Genet.
.
140
(5):413–8. DOI: 10.1002/ajmg.a.31074. PMID 16470747. S2CID 2449346. - Buntinx IM, Hennekam RC, Brouwer OF, et al (March 1995). "Clinical profile of Angelman syndrome at different ages." American Journal of Medical Genetics
.
56
(2): 176–83. DOI: 10.1002/ajmg.1320560213. PMID 7625442. - Leung, H. T.; Ring, H (January 2013). "Epilepsy in four genetically determined mental retardation syndromes." Journal of Intellectual Disability Research: JIDR
.
57
(1): 3–20. DOI: 10.1111/j.1365-2788.2011.01505.x. PMID 22142420. - Andersen WH, Rasmussen RK, Strømma P (2001). "Levels of cognitive and linguistic development in Angelman syndrome: a study of 20 children." Speech therapy, Phoniatrics, Vocology
.
26
(1): 2–9. DOI: 10.1080/140154301300109044. PMID 11432411. - Lossie A, Driscoll D (1999). "Transmission of Angelman syndrome from an affected mother". Genet Med
.
1
(6): 262–6. DOI: 10.1097/00125817-199909000-00004. PMID 11258627. - Lan LA, den Boer AT, Hennekam RC, Regnier WO, Brouwer (1996). "Angelman syndrome in adulthood." I am. J. Med. Genet
.
66
(3): 356–60. DOI:10.1002/(SICI)1096-8628(19961218)66:3 <356::AID-AJMG21>3.0.CO; 2-K. LVP: 2066/22929. PMID 9072912. - Coppus, Antonia MW (2013). "People with mental retardation: what do we know about maturity and life expectancy?" Developmental Disabilities Research Reviews
.
18
(1): 6–16. DOI: 10.1002/ddrr.1123. PMID 23949824. - Steffenburg S, Gillberg CL, Steffenburg U, Kyllerman M (1996). "Autism in Angelman syndrome: a population-based study." Pediatrician. Neurol
.
14
(2): 131–6. DOI: 10.1016/0887-8994 (96) 00011-2. PMID 8703225. - Petersen MB, Brøndum-Nielsen K, Hansen LK, Wulff K (1995). “Clinical, cytogenetic and molecular diagnosis of Angelman syndrome: estimated prevalence rate in a Danish county; the disease predominantly affects Anglo-Saxons." I am. J. Med. Genet
.
60
(3): 261–2. DOI: 10.1002/ajmg.1320600317. PMID 7573182. - Galassi FM, Armocida E, Rühli FJ (September 2016). "Angelman Syndrome in a Child Portrait with a drawing by Giovanni F. Caroto" (PDF). JAMA Pediatr
.
170
(9):831. doi:10.1001/japapediatrics.2016.0581. PMID 27380555. CS1 maint: uses the authors parameter (link) - Williams, Charles. "Harry Angelman and the AS Story". Stay up to date
. USA: Angelman Syndrome Foundation. Archived from the original on 2011-06-30. Retrieved July 1, 2011. - Dooley, J.M.; Berg JM; Pakula Z; McGregor DL. (1981). "Angelman doll syndrome." Am J Dis Child
.
135
(7):621–4. DOI: 10.1001/archpedi.1981.02130310027010. PMID 7246489. - Williams, California; Frias JL (1982). "Angelman ("happy puppet") syndrome." Am J Med Genet
.
11
(4): 453–60. DOI: 10.1002/ajmg.1320110411. PMID 7091188. - Magenis, R. E.; Brown MG; Lacy DA; Budden S; LaFranchi, S. (1987). "Angelman syndrome is an alternative outcome of del(15)(q11q13)?". Am J Med Genet
.
28
(4):829–38. DOI: 10.1002/ajmg.1320280407. PMID 3688021. - Tan, W.H.; Bird, L. M. (June 2021). "Pharmacological methods of treating Angelman syndrome." Wiener medizinische Wochenschrift
.
167
(9–10): 205–218. doi:10.1007/s10354-015-0408-Z. PMID 26758979. S2CID 5959954.
Miller-Dieker lissencephaly syndrome
In Miller–Dieker lissencephaly syndrome, the cause of pathological changes is the deletion of certain genes in the 17p13 locus. In this case, the central nervous system suffers the most. Along with lissencephaly (smoothing of the convolutions located on the surface of the brain due to disruption of the PAFAH1B1 gene), there is a reduction in the number of cortical layers. If normally there are 6 of them, then in patients only 4 can be found. Associated signs are a noticeable change in facial shape. In addition, patients grow slowly. Attempts to integrate into society are complicated by multiple pathologies of the heart, gastrointestinal tract, and kidneys. If the disease causes a deletion of the 14-3-3 epsilon gene, the syndrome becomes much more severe.
Features of education
Clinical manifestations of the disease may disappear or change with age, replacing each other. It all depends on the conditions and emotional environment in which the sick baby lives. Children suffering from this disease need special treatment from their parents .
In particular, you should never focus a child’s attention on the fact that he is sick, defective, or different from other children.
A sick child, like all other children, needs love, affection, and understanding from people dear to him.
Of course, in order to instill in a child basic skills and at least some independence, parents will have to work hard, because actions that are considered normal for other children cause serious difficulties for a sick child.
However, if you follow the recommendations of your doctor and regularly conduct special physical and educational activities with your child, the chance that your child will live a more or less comfortable life increases significantly.
Aniridia
With aniridia, the normal structure of the eye is disrupted: the organ of vision lacks the iris. In addition, concomitant pathological changes often develop, such as macular hypoplasia and optic nerve hypoplasia, corneal changes, and cataracts. Visual acuity noticeably drops, attempts at correction do not bring significant results. Photophobia and horizontal nystagmus develop. In some cases, congenital glaucoma appears.
The cause of the disease is a dysfunction of the PAX6 gene from the short arm of chromosome 11. The protein it encodes leads to the launch of a number of processes that control the process of proper formation of the visual organs and a number of other structures. It is noteworthy that the gene is very conservative: the difference between the forms of PAX6 in humans and zebrafish is less than 5%, despite the divergence of evolutionary lines approximately 400 million years ago.
The disease belongs to the group of autosomal dominant pathologies. In the case of homozygosity for a mutant copy of the PAX6 gene, the negative effect on the body increases, which causes multiple disturbances in the functioning of the visual organs. In addition, the central nervous system is affected, which leads to death.
Treatment is aimed at relieving symptoms. To visually imitate the pupil, it is recommended to use specially colored lenses. It is possible to restore the pupil through reconstructive plastic surgery.
Clinical characteristics of the child
Outwardly, a sick child looks quite content and happy, however, this impression is deceptive .
Such children always have a severe delay in mental and physical indicators, which leaves a negative imprint on learning and communication with peers.
In severe cases, a persistent and significant impairment of hand motor skills develops, which makes the child unable to take care of himself (in particular, the child cannot independently fasten a button or zipper on clothes or shoes, or perform any other actions that seem quite ordinary to a healthy person) .
All this significantly worsens the child’s quality of life.
Children suffering from Angelman syndrome also have external differences , such as:
- flat face (its middle part), insufficient development of the facial bones of the skull;
- small and pointed chin;
- hypertrophied lower jaw, which protrudes strongly forward;
- a large and wide tongue that the child often sticks out;
- violations of the dentition, the presence of interdental spaces;
- small skull (the rest of the bones of the skeleton are of normal size, corresponding to age indicators);
- deformation (curvature) of the spine.
DiGeorge syndrome
With DiGeorge syndrome, patients have a congenital form of aplasia of the parathyroid glands and thymus. It is a type of idiopathic isolated hypoparathyroidism. It is quite rare.
In this disease, pathological changes concern the parathyroid (parathyroid) glands, which exhibit dysgenesis or agenesis. The thymus gland (thymus) is absent from birth. As a result of the combination of such pathologies, a sharp decrease in the number of T-lymphocytes occurs, and immunological deficiency is formed. In addition, this syndrome is accompanied by the formation of congenital anomalies of large vessels.
The disease is autosomal and is determined by the presence of a mutation on chromosome 22. In most cases, the cause is a sporadic deletion of 22q11 (less commonly, microdeletion of 22q11.2). Inheritance occurs according to the dominant principle and is not related to gender. Some authors do not agree with this characterization and argue in favor of an autosomal recessive type with varying expressiveness.
The disease is characterized by disruption of the process of embryogenesis of 3-4 gill pouches, which leads to disruption of the thymus and parathyroid glands.
In the clinic, the most constant symptoms are candidomycosis and hypoparathyroidism, quite often accompanied by disruption of the formation of the mouth, nose and ears.
The thymus remains undeveloped due to developmental disorders in the embryonic period. The thymic epithelium does not support the normal development of T cells. As a result, a specific form of immunodeficiency is formed, in which the humoral immune response and the response at the cellular level are weakened. If a child has such a pathological disorder of immunity, he will have increased sensitivity to infections of bacterial, viral and fungal origin.
The syndrome can occur in the form of a genetically determined absence of the parathyroid glands or isolated insufficiency of the parathyroid glands - accompanied by hypocalcemic seizures that begin from birth. Immunological deficiency leads to the appearance of various infectious diseases. Typically, a combination of symptoms causes heart failure. In addition, infectious diseases cause death.
Diagnosis of the syndrome involves identifying pathologies typical of the syndrome: distortions of the shape of the face and skull, the presence of immunological deficiency, thymic aplasia, dysgenesis or agenesis of the parathyroid glands. The most pronounced manifestations of the disease are candidomycosis and hypoparathyroidism.
Forms
Angelman syndrome is associated with four types of genetic mutations:
- A newly emerged chromosomal mutation, which is associated with the loss of a section of the chromosome at locus 15 q11 - q13. This mutation is the cause of about 80% of all cases of the disease.
- Unipaternal disomy, which is associated with the loss of the maternal locus (lack of maternal genetic material). This option is rare (about 5% of all cases).
- A defect in a number of genes subject to genomic imprinting (GI). These defects occur in 2-4% of patients as a result of a direct violation of imprinting (differences in the conversion of gene information into protein or RNA, which depend on the origin of the gene). Most often occurs as a result of loss of the GI regulatory center. GI defects without loss of the regulatory center are the result of a spontaneous mutation, the repetition of which is very rare.
- A spontaneous mutation in the maternal copy that causes the brain copy of the UBE3A gene to fail to transform. This gene encodes the activity of ubiquitin ligase (an enzyme involved in the complex process of protein breakdown). Deficiency of this enzyme is one of the molecular mechanisms of the syndrome.
It is currently not possible to establish the form of the disease in 7-9%.
Retinoblastoma
Retinoblastoma is a malignant tumor of the retina. The development process usually begins in childhood, and the starting material is tissue of embryonic origin. The peak phase occurs at two years of age.
Almost all known cases are detected during the first 5 years of life.
The cause of the disease in most cases is a mutation in the genetic material. In this case, it is necessary to have a genetic condition due to the presence of a mutant version of the Rb gene, obtained by inheritance. The second tumor-causing mutation occurs in the retinoblast.
There is a small chance that parents who have had retinoblastoma may have children with no pathological changes.
There are unilateral and bilateral cases of retinoblastoma. According to statistics, for the bilateral form the probability of hereditary origin is noticeably higher.
Symptoms of the disease include eye pain, glowing pupils, and loss of vision. It is very, very difficult to identify them in a small child.
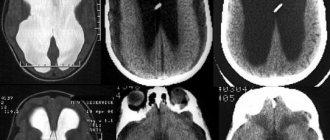
Diagnosis usually takes the form of an examination under anesthesia using ultrasound, CT and MRI. A fairly common procedure is a red bone marrow biopsy and spinal puncture. There are 5 groups based on the severity of symptoms.
There are two effective treatment methods. With cryotherapy and photocoagulation, it remains possible to preserve both vision and the eye itself. Complications with their use rarely occur. However, if a relapse occurs, the treatment will need to be repeated in the same form. Typically cryotherapy is used in cases where the anterior retina is damaged. For the posterior region, photocoagulation appears to be the preferred option.
Treatment and prevention
It is impossible to influence a genetic defect; disorders occur at the chromosomal level, so patients are prescribed symptomatic treatment and psychological correction.
Drug therapy can be used for epileptic seizures. Anti-anxiety medications may be used to alleviate symptoms and improve sleep.
Individual programs are developed for each patient - exercise therapy, speech therapy classes, therapeutic massage, behavioral therapy. They provide an opportunity to improve a person’s quality of life. Speech therapists, speech pathologists, specialists in non-verbal communication and behavioral therapy are also involved.
For preventive purposes, couples planning to conceive a child are advised to consult a geneticist.