A child can get diabetes at any age. But most often the pathology develops at 5-8 years and at 10-12 years. It is during these periods that increased growth of the body is observed, which is accompanied by intense metabolism. But recently, the number of diabetics among children under 5 years of age, including newborns, has been increasing.
Types of diabetes
- Type 1 – insulin dependent.
- Type 2 – insulin-independent.
The first type is most common in children. The disease is characterized by very low levels of insulin; the child requires constant monitoring.
In type 1 diabetes, the body does not produce enough insulin; it is necessary to constantly monitor its level and administer additional insulin if necessary. The cause is unknown and the disease cannot be prevented. Symptoms include thirst, constant hunger, excessive urination, fatigue, decreased visual acuity, and weight loss. Symptoms may occur suddenly.
Type 2 diabetes was previously observed only in adults, but is now appearing in children as well. The pathology is a consequence of ineffective absorption of insulin by the body. Develops against a background of physical inactivity and excess weight. Symptoms are less pronounced than with type 1. Source: A.B. Resnenko Type 2 diabetes mellitus in children and adolescents: from pathogenesis to treatment // Pediatric pharmacology, 2011, vol. 8, no. 4, pp. 125-129
Comparison table of LED types
| Signs and symptoms | Type 1 diabetes | Type 2 diabetes |
| The essence of metabolic processes | Insulin deficiency | Decreased insulin production |
| Spreading | In 5-10% of cases | In 90-95% of cases |
| Patient age | Children and teenagers | People over 40 years old |
| Body Features | Normal | Obesity |
| Onset of the disease | Acute | Unnoticeable, gradual |
| The body produces insulin | β-cells are affected, production is stopped | Insulin is produced, but cells do not use it to carry sugar |
| Insulin use | Necessarily | Not necessary |
| Treatment options | Insulin administration, diet | Diet, increase physical activity, take pills to reduce sugar |
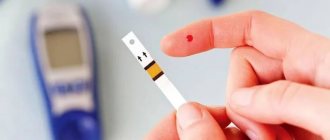
Carrying out analysis
If a child has type 2 diabetes, then it is necessary to check the blood sugar level 1-2 times a day, but if the child does not have type 2 diabetes, then it will be necessary to check slightly more often. The more often a sugar test is performed, the more a person learns about his condition, as well as what level sugar is usually in the body.
How to properly perform a blood sugar test:
- Wash your hands clean;
- Replace the needle in the device;
- Take a new strip from the case, after taking out the strip, you need to close the case as quickly as possible to avoid damaging the remaining strips;
- Prepare a glucometer;
- Make a puncture in your finger;
- Drop some blood onto a special strip;
- Stop the bleeding;
- Wait for the end of the study, view the result, and then write it down in a specialized form.
Where does the disease come from in children?
Each type of diabetes has its own causes. There are risk factors that provoke the disease:
- mother or both parents are diabetic;
- viruses;
- high birth weight (more than 4.5 kg);
- obesity;
- presence of diathesis;
- frequent colds;
- weakened immune system;
- metabolic disease.
Many people are concerned about whether the disease is transmittable. The main cause of type 1 diabetes in children is heredity. This is confirmed by the large number of family cases when the disease occurs in a child in the presence of pathology in parents, grandparents.
The autoimmune process is initiated by an external factor – viruses. Chronic insulitis and insulin deficiency are caused by exposure to the ECHO virus, Coxsackie B virus, herpes, rubella, and Epstein-Barr. The disease can be caused by enterovirus, rotavirus, measles and others. Source: P.F. Litvitsky, L.D. Maltseva Disorders of carbohydrate metabolism in children: diabetes mellitus // Issues of modern pediatrics, 2021, v. 16, no. 6, p. 468-480
In children with a genetic predisposition, the disease can be triggered by intoxication, artificial feeding, monotonous carbohydrate diet, as well as stress and previous operations.
Type 2 diabetes in children develops due to dysfunction of the pancreas - when b-cells do not produce or secrete insulin. As a result, the sensitivity of the receptors decreases.
The order of development of pathology
Insulin is a hormone produced by the pancreas, which is small in a child. By the age of 10, the iron weighs 50 grams and its length is 12 cm. The main function is the production of insulin. The gland should cope with the task by the age of 5.
The greatest risk of diabetes occurs between the ages of 5 and 11 years. At this time, metabolism is fast, sugar is well absorbed. Every child needs 10 grams of carbohydrates. The exchange is controlled by an incompletely formed nervous system, and failures may occur.
Those at risk include teenagers going through puberty, premature babies and children who experience heavy stress.
The course of the disease depends on the age at which the pathology appeared. In children, diabetes is severe and leads to complications. Parents must understand that diabetes in children requires care and lifelong treatment.
Parental responsibilities
Regardless of the reason for the increase in sugar, the child needs treatment. The responsibilities of parents include creating comfortable living conditions and constant monitoring of the therapy. Necessary:
- purchase a glucometer with test strips and kitchen scales;
- monitor glycemia several times a day;
- do not violate the insulin treatment regimen;
- organize proper nutrition and systematic sports activities;
- regularly take your child to an endocrinologist for control and preventive examinations;
- provide psychological support and assistance.
To facilitate adaptation to the disease, endocrinologists strongly recommend attending classes at the Diabetes School. Children with a hereditary predisposition to diabetes need to begin prevention from the moment they are born. A child prone to hyperglycemia or diagnosed with diabetes needs to be properly explained which foods are strictly contraindicated for him and for what reason.
Symptoms and first signs
The disease can develop in a child at any age. There are two peak periods - 5-8 years and puberty, when growth and metabolism are enhanced.
The disease of the first type manifests itself acutely. Signs of type 1 diabetes in children are : severe weakness and dizziness when hungry and full . From the onset of the first symptoms to a diabetic coma can take from 1 to 3 months. Source: ISPAD Clinical Practice Consensus Guidelines 2009 Compendium. Pediatric Diabetes. 2009; 10 (Suppl. 12): 210
The first signs of the development of diabetes mellitus in children:
- increased urination (over 2 liters per day);
- thirst and dry mouth;
- increased appetite with a sharp decrease in body weight;
- severe course of infectious diseases;
- rapid fatigue without exercise;
- absent-minded attention;
- increased blood glucose (exceeds 120 mg) in the analysis;
- rapid decrease in visual acuity;
- nausea and vomiting.
Parents may notice that the urine has become sticky, and starched stains remain on their underwear. Dryness of the mucous membranes and epidermis may be observed - peeling of the skin on the soles and palms. Symptoms of diabetes mellitus in children include irritation in the corners of the mouth (“seizures”) and stomatitis. Pustules, boils, and diaper rash appear. In girls, the development of the disease is accompanied by vulvitis, in boys – by balanoposthitis. If the disease first appears during a girl’s puberty, it can cause menstrual irregularities.
It is difficult to identify symptoms in children, so they pay attention to accompanying manifestations. Young children are characterized by nocturnal enuresis, itching, restlessness, and digestive problems. When sick, an infant greedily drinks milk and water. The sweet, sticky urine makes the diapers hard. Such signs indicate a moderate to severe form of pathology. In mild forms, the disease is diagnosed by blood and urine tests.
Traditionally, all cases of childhood diabetes mellitus (DM) with polydipsia, polyuria and weight loss against the background of elevated glycemic levels are classified as type 1 (autoimmune). But today, more and more often, children are diagnosed with type 2 diabetes and other specific types (type 3 diabetes). It is estimated that the true prevalence of “non-type 1 diabetes” in childhood may reach 10% (against the background of the increasing incidence of type 1 diabetes). At the beginning of 2021 in Belarus, approximately 260 children with diabetes may have non-immune, including monogenic forms. With the development of molecular genetics, endocrinologists have a real opportunity to diagnose many variants of this disease.
MODY: adult-onset diabetes mellitus in young people
Anzhelika Solntseva, Director of the Republican Scientific and Practical Center for Pediatric Oncology, Hematology and Immunology, Doctor of Medicine. Sciences, professor. Monogenic forms of diabetes in children are caused by certain defects in the function of islet cells of the pancreas or the action of insulin. In 1974, Robert Tattersall and colleagues described a case of mild diabetes with a dominant type of inheritance in members of one family, proposing the name “maturity-onset–type diabetes of young people (MODY)”, the genetic causes of which were identified later: in 1992, a mutation in the glucokinase gene (GCK) was discovered, in 1996 - in hepatocyte nuclear factor (HNF) and HNF1a, in 1997 - in the insulin promoter factor and HNF1b.
MODY is a heterogeneous group of diseases with an autosomal dominant pattern of inheritance that are caused by mutations in genes involved in the synthesis and secretion of insulin. MODY is the most common group of monogenic forms of diabetes. Its subtypes are often designated by the mutant gene (eg, GCK-MODY). There are 14 known subtypes of MODY, which account for 1–2% of all cases of diabetes. The prevalence of MODY ranges from 21–45:1,000,000 cases in children and 100:1,000,000 in adults.
The presence of MODY can be assumed:
1. In patients diagnosed with type 1 diabetes:
- with a family history of diabetes of any type;
- in the absence of antibodies to components of pancreatic islet cells;
- with preserved insulin secretion according to the study of C-peptide levels;
- with a low need for insulin (less than 0.5 units/kg/day) and in the absence of the development of ketoacidosis after skipping injections outside the honeymoon period (5 or more years from the diagnosis of diabetes).
2. In patients with diagnosed type 2 diabetes in the absence of signs of insulin resistance (obesity, acanthosis nigricans, increased triglyceride levels).
The diagnosis of the disease is confirmed by the results of a molecular genetic study. In most cases, treatment for MODY differs from diabetes types 1 and 2, so verification of the diagnosis allows you to select the optimal therapy. Characteristics of MODY subtypes are given in the table.
The most common MODY subtypes are GCK-MODY (MODY 2), HNF4A and HNF1A (MODY 1 and 3). Since June 2021, in the clinical diagnostic laboratory of the Republican Scientific and Practical Center for Pediatric Oncology, Hematology and Immunology (headed by Candidate of Biological Sciences Natalia Lipai), a method for diagnosing mutations in the exons of the HNF4A, GCK, HNF1A, HNF1B genes has been developed and introduced into clinical practice.
Regulation of insulin secretion
The enzyme glucokinase is a kind of cellular glucose sensor. It catalyzes the ATP-dependent phosphorylation of glucose into glucose-6-phosphate, the first, rate-limiting reaction of glycolysis in the liver and pancreas. When the level of glycemia increases, glucose enters the cells using the high-throughput glucose transporter GLUT-2, which ensures rapid equalization of its concentration inside and outside the cells. An increase in glucose levels in the cell stimulates the irreversible reaction of glucose-6-phosphate synthesis. As a result of glucose metabolism in islet cells, the ATP/ADP ratio increases and ATP-dependent potassium channels close. The accumulation of potassium ions inside the cell causes depolarization of the membrane and the opening of calcium channels. Calcium ions entering the cell stimulate insulin secretion (see figure).
Drawing . Regulation of insulin secretion
Clinical picture of GCK-MODY (MODY 2)
To date, more than 800 mutations of the glucokinase gene have been described that impair the function of the enzyme. Carriers of the homozygous mutation develop permanent neonatal diabetes. Heterozygous carriage leads to partial glucokinase deficiency. Fasting glucose concentrations increase due to the liver's reduced ability to synthesize glycogen, since glucose phosphorylation is the first reaction of this process.
Laboratory findings suggestive of GCK-MODY in a patient:
1) persistent mild asymptomatic fasting hyperglycemia (5.6–8.5 mmol/l);
2) moderate increase in glycemia during OGTT. 2 hours after exercise, glucose concentration increases by less than 3 mmol/l in 70% of patients and by no more than 4.6 mmol/l in 90% of cases;
3) a slight increase in the level of glycated hemoglobin HbA1c in the range of 5.6–7.6% (with a higher value the diagnosis is unlikely);
4) preserved production of endogenous insulin (C-peptide level after meals more than 200 pmol/l);
5) the presence of mild hyperglycemia in either parent.
Most often, GCK-MODY is detected during a random or screening examination during pregnancy. It is believed that 40–50% of children with incidentally reported asymptomatic hyperglycemia have this subtype of MODY. To verify the diagnosis, molecular genetic confirmation is necessary.
Table. Characteristics of MODY subtypes
GCK-MODY Treatment
With GCK-MODY, hyperglycemia does not reach high levels, impaired insulin production does not progress, so micro- and macrovascular complications rarely develop. Proteinuria, proliferative retinopathy, and neuropathy occur in no more than 4–6% of patients with GCK-MODY. Drug treatment is not required for patients with this subtype of diabetes, with the exception of pregnant women. Moreover, therapy with insulin and oral hypoglycemic drugs does not always improve carbohydrate metabolism. Prescribing small doses of insulin leads to a compensatory decrease in the production of endogenous insulin, and as a result, the content of the hormone in the body will remain unchanged. A decrease in glucose concentration occurs only with the introduction of supraphysiological doses of the drug. Insulin therapy is recommended only for pregnant women in cases where the fetus has not inherited the GCK gene mutation.
A carbohydrate-restricted diet has a moderate effect on the average daily level of glycemia by reducing its postprandial (after eating) rises.
Clinical picture of MODY with disruption of transcription factors HNF4A and HNF1A (MODY 1 and 3)
Mutations of the HNF1A and HNF4A genes lead to impaired expression of proteins involved in the transport and metabolism of glucose, synthesis and secretion of insulin. These MODY subtypes develop during puberty or postpuberty and are characterized by decreased fasting and postprandial insulin secretion. The progressive deterioration of pancreatic b-cell function is associated with their reduced proliferation and accelerated apoptosis. Distinctive features of HNF4A-MODY are macrosomia at birth (in 56% of cases) and hyperinsulinemic hypoglycemia in the neonatal period (in 15% of patients). Patients with HNF1A/HNF4A-MODY are characterized by high sensitivity to sulfonylureas. Insulin secretion decreases by 1–4% annually, so after a few years patients become resistant to sulfonylureas and require insulin therapy. When using sulfonylureas, patients are at high risk of hypoglycemic conditions.
Characteristic features of MODY with disruption of transcription factors:
- development during puberty or post-puberty;
- Diabetes is preceded by impaired glucose tolerance, which is confirmed by a significant (more than 5 mmol/l) increase in glycemia 2 hours after oral glucose intake;
- fasting glucose remains normal for a long time due to residual insulin production;
- ketoacidosis is not typical.
The risk of developing microvascular complications depends on the quality of glycemic control.
Treatment of MODY with Transcription Factor Disorder
One of the important features of HNF1A/HNF4A-MODY is its high sensitivity to sulfonylureas in the early stages of the disease, with preserved secretion of endogenous insulin. Sulfonylureas stimulate insulin secretion by binding to the sulfonylurea receptor (SUR1), a subunit of the ATP-dependent potassium channel. Their interaction leads to the closure of the potassium channel, depolarization of the membrane, the opening of calcium channels and the release of insulin.
Case from practice
Boy, 10 years old. Child from the 3rd pregnancy, delivery at 37 weeks of gestation, birth weight 3,400 g (corresponds to the 90th percentile).
The family history is burdened: type 1 diabetes in a paternal uncle since the age of 6 years, the mother has gestational diabetes, an older sister at the age of 9 was diagnosed with impaired fasting glycemia, and an older brother was diagnosed with diabetes at the age of 6.
At 4 months of life, the child first registered hyperglycemia of 6.8 mmol/l 4 hours after eating; No ketones or glucose were detected in the urine. Glucose level 2 hours after eating is 6.6 mmol/l. A diagnosis of impaired fasting glucose was made, and glycemic control and a diet limited in easily digestible carbohydrates were recommended.
Glycemic profile indicators over the next year of life ranged from 5.0–6.1 mmol/L on an empty stomach and 4.9–7.0 mmol/L after meals. There was no glucose or ketones in the urine. The level of glycated hemoglobin did not exceed the reference values - 5.7% (normal 4.0–6.2%). Hormonal examination revealed euthyroidism, a moderate decrease in the level of insulin 2.0 µU/ml (normal 2.6–25.0 µU/ml) and C-peptide - 51.7 pmol/l (normal 160–1,100 pmol/l) , normal values of antibodies to glutamate decarboxylase (GAD) are 0.75 IU/ml (normal is less than 1.0 IU/ml).
At the age of 2 years, glycemic indicators during the control OGTT were within the normal range: on an empty stomach 5.0 mmol/l, after 2 hours 4.7 mmol/l. Insulin and C-peptide levels were within the reference values: 3.95 μU/ml and 531.6 pmol/l.
Over the next 4 years, while following the diet, normoglycemia was noted, HbA1c levels ranged from 5.5–5.9%.
At the age of 6, the child began to complain of lethargy and weakness. No weight loss was noted: over the previous 5 months the boy gained 1 kg. During laboratory examination, the cito glycemic level was 4.7 mmol/l. A urine test revealed 4+ ketonuria, no glucose detected. The child was urgently hospitalized at the Republican Children's Endocrinology Center.
On admission the condition was of moderate severity. Body weight 19 kg, height 115.5 cm (standard deviation coefficient (SDS) -0.4), BMI 14.2 kg/m2 (-0.83 SDS). Physical development is average, harmonious. When examining the acid-base state, there were no features. The child received infusion therapy with saline solution, Ringer's solution and 5% glucose, and was prescribed table D with a restriction of easily digestible carbohydrates.
In glycemic profiles: on an empty stomach 4.5–6.1 mmol/l, during the day 5.3–7.5 mmol/l. A moderate decrease in the levels of insulin (2.36 µU/ml) and C-peptide (89.49 pmol/l) on an empty stomach was detected with normal values 2 hours after a glucose load: insulin 3.5 µU/ml, C-peptide 320.5 pmol/l. The values of antibodies to GAD (0.25 IU/ml) and glycated hemoglobin (5.8%) were within the reference parameters. Ultrasound of the abdominal organs and thyroid gland - without pathology. The child was examined by a urologist: adhesive balanoposthitis.
The diagnosis was made: unspecified diabetes; monogenic type of diabetes cannot be excluded; ketosis.
The patient was discharged as an outpatient with recommendations to follow a diet limited in rapidly digestible carbohydrates.
In the following years, the indicators of carbohydrate metabolism corresponded to the metabolic compensation of the disease: HbA1c within 5.8–6.4%. No further episodes of ketosis were recorded.
Currently, the boy's height is 136 cm (-0.28 SDS), body weight 30 kg, BMI 16.2 kg/m2 (-0.2 SDS). Physical development is average, harmonious. Height corresponds to genetic (175 cm, -0.2 SDS). He is not receiving medication treatment. Fasting glycemia is in the range of 5.4–6.5 mmol/l, during the day 6.5–7.9 mmol/l, HbA1c 6.4%.
The boy was diagnosed with 2 heterozygous mutations: in the GCK gene and the HNF4A transcription factor gene. Considering the presence of diabetes in several family members, a molecular genetic examination of siblings and their parents was carried out for monogenic types of diabetes. Different combinations of 3 types of mutations were found in children: in the glucokinase gene (GCK-MODY, or MODY 2), the transcription factor HNF4A (HNF4A-MODY, or MODY 1) and the insulin receptor (INSR). The mother was found to be a heterozygous carrier of mutations in the GCK and HNF4A genes, and the father was found to be a heterozygous carrier of mutations in the INSR gene.
Final diagnosis: monogenic diabetes mellitus (GCK- and HNF4A-MODY).
Diagnosis of diabetes mellitus
The first person to identify symptoms of the disease is the pediatrician who observes the child. The doctor pays attention to the classic signs: increased urination, feelings of thirst, hunger and weight loss.
During the examination, the doctor may notice diabetic blush, decreased skin turgor and a crimson tongue. If symptoms of diabetes are detected, the pediatrician transfers the patient to an endocrinologist for treatment and observation.
To make an accurate diagnosis, the child is sent for laboratory testing. It is necessary to take a blood test to check your sugar level; daily monitoring is used. They also check insulin, proinsulin, glucose tolerance, level of glycosylated hemoglobin, blood CBS.
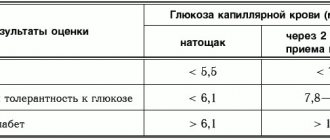
Blood test results table
| results | Capillary blood glucose (mmol/l) | |
| on an empty stomach | 2 hours after taking glucose | |
| Norm | <5,5 | <7,8 |
| Impaired glucose tolerance | <6,1 | 7,8-11,1 |
| Diabetes | >6,1 | >11,1 |
The urine is tested for glucose and ketone bodies. The criteria for identifying the disease are: glycosuria, hyperglycemia, acetonuria, ketonuria.
As part of the preclinical detection of type 1 pathology in genetically predisposed children, Abs to glutamate decaboxylase and β-cells are determined. The structure of the gland is assessed using ultrasound.
Based on the patient’s complaints, urine and blood test results, and research results, the doctor makes a diagnosis.
How to write down the result correctly
The main thing in monitoring blood sugar levels is the right approach and timely recording of the results. In order for the attending physician to more accurately determine treatment, he needs to see how the sugar level fluctuates throughout the day, this will help determine the correct dose of insulin.
The main thing is not to forget to take the results of the self-test when going to the doctor. It is advisable to use a glucometer that has a function for storing blood test results. There are a huge variety of such models, there are even those that store up to 100 transactions.
- Conversation on the topic of diabetes prevention
Treatment of the disease
When a diagnosis is made, the doctor, in accordance with clinical recommendations, prescribes observation and examination of the child every month. Monitoring the condition allows you to make adjustments to therapy, prevent exacerbations and prevent the pathology from becoming severe.
Treatment of diabetes in children includes medications, special exercise and diet. Source: I.I. Dedov, V.A. Peterkova, T.L. Kuraeva Russian consensus on the treatment of diabetes mellitus in children and adolescents // Pediatrics, 2010, v. 89, no. 5, pp. 6-14
- Proper nutrition. The specialist develops a balanced diet in terms of calories and nutritional supplements. A complex of vitamins is required. The diet should limit the consumption of baked goods and potato dishes. Unsweetened fruits and vegetables can be eaten in any quantity. The child needs to be provided with 6 fractional meals a day.
- Exercise helps lower glucose levels and improve insulin sensitivity. The load must be accurately dosed and selected by the attending physician. During and after exercise, you need to take carbohydrates.
- Medicines. Children are prescribed medications with insulin. In most cases, a single dose every day is sufficient. The doctor selects the dose and administration schedule. In addition to the main treatment or for mild diabetes mellitus in children, tablets are indicated.
The main tests for detecting type 1 diabetes
- Glucose (code: 23-12-001). Increased blood glucose levels - hyperglycemia indicates insulin deficiency.
- C-peptide (code: 33-20-010). A low level of C-peptide indicates that insulin is not being produced by pancreatic cells.
- Antibodies to insulin (code: 52-20-209). Elevated levels are observed in 100% of children within five years from the onset of insulin-dependent diabetes.
- Antibodies to the islets of Langerhans (code: 52-20-207). The presence of antibodies to the islets of Langerhans (located in the pancreas) in the blood serum allows one to diagnose diabetes long before the onset of clinical symptoms, which means prescribing a diet and selecting effective medications.
Disease prevention
No specific prevention has been developed for children with diabetes. For people at risk, it is important to maintain a normal weight, ensure daily physical activity, improve immunity, and be examined by an endocrinologist twice a year.
Timely vaccination helps prevent the development of diseases that are caused by diabetes: measles, mumps.
It is necessary to ensure that the child drinks sufficient amounts of liquid - at least 2 liters, in addition to tea and juices.
It is necessary to protect the child from stress; if there is excessive anxiety, it is better to consult a specialist.
Ways to reduce indicators
Hyperglycemia in children is compensated, first of all, by adjusting the diet. The condition of impaired glucose tolerance (prediabetes) is reversible. To prevent the development of diabetes, it is enough to review your diet and diet. If sugar levels are high, it is recommended to switch the child to a diet designed for diabetics.
If the juvenile type of the disease is confirmed, the child will receive lifelong treatment with medical insulin and a diabetic diet. The dosage of medications and the treatment regimen are determined by an endocrinologist. Insulin injections are carried out according to an individual schedule determined by the doctor. Short- and long-acting medical insulins are used for treatment.
Nutrition
A small patient is prescribed the “Table No. 9” diet, which helps maintain a stable glycemic level and prevent the early development of diabetic complications. Products containing large amounts of fast carbohydrates should be excluded from the menu:
- ice cream, cakes and other confectionery products;
- sweet pastries, jam, sweets;
- fruits: papaya, guava, carom, bananas, pineapples, figs;
- drinks: packaged juices, sweet soda, bottled tea.
The menu is based on protein products (dietary poultry, fish, mushrooms, eggs) and complex carbohydrates, which are slowly processed in the body. Slow carbohydrates include legumes, grains, and vegetables. Potatoes are allowed on a limited basis.
All foods for the diet are selected taking into account their glycemic index (GI), which indicates the rate at which glucose formed during the digestion of food enters the blood. In case of hyperglycemia, foods indexed from 0 to 30 are allowed; foods with an index from 30 to 70 are limited. A glycemic index of more than 70 is not allowed on the menu.
Brief table of products with index
Physical activity
A prerequisite for the treatment of diabetes and pre-diabetic conditions is participation in feasible sports. The load must correspond to the child’s physical capabilities and have a regular basis.
Effective treatment at SM-Clinic
Our medical center employs the best endocrinologists in St. Petersburg. At an appointment with a specialist, you will receive qualified assistance - we will accurately diagnose and prescribe treatment. To schedule a consultation, call us at the numbers provided. The doctor will answer your questions about the diagnosis, treatment and prevention of diabetes.
Sources:
- A.B. Resnenko. Type 2 diabetes mellitus in children and adolescents: from pathogenesis to treatment // Pediatric pharmacology, 2011, vol. 8, no. 4, pp. 125-129.
- P.F. Litvitsky, L.D. Maltseva. Disorders of carbohydrate metabolism in children: diabetes mellitus // Issues of modern pediatrics, 2021, vol. 16, no. 6, pp. 468-480.
- ISPAD Clinical Practice Consensus Guidelines 2009 Compendium. Pediatric Diabetes. 2009; 10 (Suppl. 12): 210.
- I.I. Dedov, V.A. Peterkova, T.L. Kuraeva. Russian consensus on the treatment of diabetes mellitus in children and adolescents // Pediatrics, 2010, v. 89, no. 5, pp. 6-14.
Astratenkov Oleg Gennadievich Clinic Author of the article
Astratenkov Oleg Gennadievich
Doctor of the highest qualification category
Specialty: endocrinologist
Experience: 19 years
The information in this article is provided for reference purposes and does not replace advice from a qualified professional. Don't self-medicate! At the first signs of illness, you should consult a doctor.
Prices
| Name of service (price list incomplete) | Price |
| Appointment (examination, consultation) with a pediatric endocrinologist, primary, therapeutic and diagnostic, outpatient | 1750 rub. |
| Consultation (interpretation) with analyzes from third parties | 2250 rub. |
| Prescription of treatment regimen (for up to 1 month) | 1800 rub. |
| Prescription of treatment regimen (for a period of 1 month) | 2700 rub. |
| Consultation with a candidate of medical sciences | 2500 rub. |