Acute myeloid leukemia (the most commonly used abbreviation is AML) is a malignant pathology of the blood and bone marrow, characterized by the uncontrolled production of immature (blast) myelocytic cells - red blood cells, granulocytes, neutrophilic leukocytes, platelets. The disease is diagnosed in cases where more than 20% of immature cells are detected in the myelogram. Its characteristic feature is its rapid course: within a few months, immature cells begin to predominate in the composition of the blood, which is why it ceases to perform its main functions. The patient's condition deteriorates sharply, and in the absence of treatment, the prognosis is extremely unfavorable.
Kinds
Oncohematologists distinguish several dozen types of acute myeloid leukemia, which are grouped according to similar characteristics:
- with typical genetic mutations;
- with dysplasia that causes cell changes;
- arising as a result of treatment of other diseases;
- with the proliferation of myeloid germ against the background of Down syndrome;
- myeloid sarcomas;
- dendritic cell plasmacytoid tumors.
Depending on the form of the disease, the doctor chooses treatment tactics, since the duration of remissions and the overall prognosis for different types have serious differences.
Pathophysiology
Under the influence of the above etiological factors, somatic mutations of the precursor cells of hematopoietic and lymphoid tissues occur in the human body.
https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1526-0968.2002.00402.x
The first stage of leukemia formation begins with a mutation in the parent cell, which acquires the ability to rapidly proliferate. As a result of this process, cells are formed that are their clone.
At the stage of formation of the first copy, the tumor retains the ability to further differentiate (benign tumor growth). Over time, numerous mutations and changes in structure occur in the primary leukemia clone. As a result, they not only actively proliferate, but also lose the ability to differentiate (malignant tumor growth).
In both children and adults, acute leukemia can present with extremely high blast counts; a phenomenon known as hyperleukocytosis. Respiratory failure, intracranial hemorrhage and severe metabolic abnormalities frequently occur in acute hyperleukocytic leukemia (AHL) and are the main determinants of the observed high early mortality (20% to 40%).
The process leading to these complications has long been known as leukostasis, but the biological mechanisms underlying its development and progression remain unclear. Traditionally, leukostasis is associated with the accumulation of leukemic blasts in the microcirculation, and its treatment is aimed at quickly reducing leukocytosis. https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1526-0968.2002.00402.x
Symptoms
In the initial stage of acute myeloid leukemia, there are practically no symptoms; as the disease progresses, the patient develops:
- pale skin and other signs of anemia;
- bleeding from the nose and gums, numerous bruises on the skin;
- elevated temperature within 37-38 degrees (subfebrile);
- profuse sweating at night;
- rash on the skin in the form of small reddish spots;
- shortness of breath even after light exertion;
- constant pain in bones and joints;
- frequent colds and other infections;
- enlarged lymph nodes;
- sudden weight loss for no apparent reason.
Cost of treatment for acute myeloid (granulocytic) leukemia in Israel
The price for treatment of acute myeloid leukemia in Israel includes the cost of all diagnostic and therapeutic procedures prescribed by the doctor. Therefore, the exact cost of treatment for acute granulocytic leukemia in Israel can only be calculated after the patient arrives at the clinic and undergoes diagnostics.
To receive information about the cost of certain medical services at the Ikhilov Complex clinic, leave your request on our website.
Advantages of treatment of acute myeloid (granulocytic) leukemia in the foreign clinic Ichilov Complex
- Patients are treated by highly qualified doctors, whose experience allows them to solve the most complex medical problems.
- The clinic’s equipment puts it a step above most medical institutions around the world. Thanks to the technical equipment of the clinic, the most complex operations are carried out within its walls, experimental therapeutic techniques and high-precision diagnostics are implemented.
- The clinic treats a wide range of diseases in patients of different ages, including the treatment of acute myeloid (granulocytic) leukemia in children in Israel.
- A patient-friendly step-by-step treatment payment system.
- Each patient has the right to choose his own attending physician.
- Patients are surrounded by the care of experienced and responsible medical staff.
- Availability of highly qualified specialists who provide patients with the necessary psychological assistance.
- 5
- 4
- 3
- 2
- 1
(8 votes, average: 5 out of 5)
Causes and risk factors
It is currently unknown what exactly influences induce blood germs to malignant mutations, but oncologists have well studied the factors that contribute to the development of acute myeloid leukemia.
- Exposure to carcinogens. Certain chemical compounds can induce cells to change. These are a number of products formed during tobacco smoking and incomplete oxidation of fats, many types of industrial emissions, some medications, etc.
- Radiation. X-rays and other radioactive, ionizing radiation change the hereditary apparatus of living cells.
- Genetic factor. The risk of the disease increases several times for people who have close relatives with leukemia.
- Diseases. Some diseases increase the likelihood of blood cancer. These are Down syndrome, congenital anemia and thrombocytopenia, neurofibromatosis, etc.
Causes of acute lymphoblastic leukemia
Risk factors are things that affect the likelihood of getting a disease. Some of them can be influenced, others cannot. Refers to several known risk factors for acute lymphocytic leukemia.
- Exposure to high levels of radiation. Treating cancer with radiation therapy also increases the risk of leukemia, especially if radiation is used in combination with chemotherapy.
- The effects of certain chemicals, such as benzene and certain chemotherapy drugs.
- Some viral infections: human T-cell leukemia virus (HTLV-1) or Epstein-Barr virus (EBV).
- Hereditary factor. Acute lymphoblastic leukemia is not a hereditary disease. But there are syndromes of genetic origin that increase the risk of the disease: Down syndrome, Klinefelter syndrome, Bloom syndrome, Louis-Bar syndrome (ataxia-telangiectasia) and Fanconi anemia.
- Ethnicity: Acute lymphocytic leukemia is more common in whites than in African Americans.
- Gender: The disease is slightly more common in men than in women.
- Having a twin increases your risk in the first year of life.
- Uncertain, unproven, or controversial risk factors: exposure to electromagnetic fields (for example, living near power lines or using cell phones); workplace exposure to chemicals (diesel fuel, gasoline, pesticides); smoking; effect of hair dye.
The causes of acute lymphoblastic leukemia are currently unknown. The likelihood of many types of cancer can be reduced by making lifestyle changes to avoid certain risk factors. But there is no known way to prevent most cases of leukemia. The majority of patients do not have the above-mentioned risk factors.
Diagnostics
Laboratory tests of blood and bone marrow samples are the main ways to diagnose acute myeloid leukemia. This:
- general peripheral blood test;
- myelogram;
- cytochemical analysis of immature cells;
- immunophenotyping;
- cytogenetic analysis;
- molecular genetic analysis;
- HLA typing;
- blood chemistry;
- Reberg's test;
- coagulogram.
In addition, the patient is prescribed instrumental examinations of internal organs to assess their condition: ultrasound of the abdominal cavity, ECG, chest x-ray. Subsequently, other tests and studies may be prescribed.
Prognosis for acute lymphoblastic leukemia
A number of studies related to the treatment of leukemia have been aimed at finding out why some people are more likely to get rid of the disease than others. Differences were found, which were called prognostic factors. They help doctors decide how much treatment to treat for a particular type of leukemia.
- Age: Younger patients have a better prognosis.
- Initial white blood cell count: A low level at diagnosis provides a better prognosis.
- Acute lymphocytic leukemia subtype: T-cell leukemia has a better prognosis than B-cell leukemia.
- Genetic mutations. Translocations between 4 and 11, and between 9 and 22 (unless targeted therapy is used), carry a worse prognosis than the absence of chromosome 7 or the presence of an additional chromosome 8.
- Response to chemotherapy treatment: Patients who achieve complete remission in the fourth to fifth week after starting treatment have a better prognosis.
In addition to the above, the status of the disease after treatment and how well the disease responded to therapy have an impact.
- Remission is a state when there are no symptoms of the disease. In the bone marrow, the rate of lymphoblastic cells is less than 5%, the level of leukocytes is within normal limits. Molecular remission is confirmed by laboratory diagnostics - PCR.
- Minimal residual disease indicates a condition in which standard laboratory tests do not detect leukemia cells, but cytometry or PCR does. Patients with this status have a risk of relapse and a poorer prognosis.
- Active disease indicates evidence of the presence of leukemia or the likelihood of relapse after therapy.
Active clinical trials of new methods for treating leukemia are underway in Israel. There is an opportunity for patients to participate.
Treatment
The main treatment method for acute myeloid leukemia is chemotherapy, which is carried out in two stages:
- induction - reducing the number of immature cells to achieve remission;
- consolidation - maximum prolongation of remission, prevention of relapses, elimination of residual effects.
Intensive therapy used during the induction phase allows achieving remission in approximately 70% of clinical cases. But treatment should absolutely not end there, since without a consolidation program, patients almost always experience a relapse after some time.
The consolidation stage, depending on the patient’s condition, includes up to five courses of chemotherapy. If the risk of relapse is high enough, a bone marrow and/or hematopoietic stem cell transplant is performed. The best donors are siblings, especially identical twins.
In addition, patients are often prescribed the administration of red blood cells and transfusion therapy (transfusions of blood and its drugs). Treatment tactics are usually chosen according to the patient's age. Remission is considered achieved if the number of myeloid cells in the bone marrow can be reduced to 20% or less.
charitable foundation
The essence of the disease
Leukemia, or leukemia, is a disease of the bone marrow, sometimes colloquially called “blood cancer.” In leukemia, normal hematopoiesis is disrupted: an excessive number of abnormal immature blood cells, usually the precursors of white blood cells, are produced. These blast cells, multiplying and accumulating in the bone marrow, interfere with the production and functioning of normal blood cells, which causes the main symptoms of the disease.
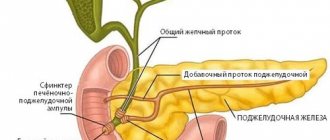
As you know, different blood cells develop differently and have different precursors - that is, they belong to different lines of hematopoiesis (see diagram in the article “Hematopoiesis”). The line of hematopoiesis leading to the appearance of lymphocytes is called lymphoid; the remaining leukocytes and other blood cells belong to the myeloid lineage. Accordingly, leukemias are distinguished from the precursor cells of lymphocytes (such leukemias are called lymphoblastic, lymphocytic, or simply lymphocytic leukemias) and from the precursors of other cells (such leukemias are called myeloblastic, myeloid, or simply myeloid leukemias).
Acute myeloid leukemia (AML, acute myeloid leukemia, acute myeloid leukemia, acute non-lymphoblastic leukemia) is a disease that is relatively rare in children, but its frequency increases with age. The term "acute" refers to the rapid progression of the disease, as opposed to chronic leukemia. The term “myeloid” (see above) means that the immature cells that form the basis of the disease belong to the so-called myeloid lineage of hematopoiesis. These cells are usually myeloblasts and their descendants, but the presence of other types of blast cells is also possible.
Within the framework of the French-American-British morphological classification (FAB), 8 main variants of AML are distinguished.
* M0 (AML with minimal differentiation): <5% of all AML cases * M1 (AML without maturation): 15-20% * M2 (AML with signs of maturation): 20-30% * M3 (Acute promyelocytic leukemia): 10- 15% * M4 (Acute myelomonocytic leukemia): 20-25% * M5 (Acute monocytic leukemia): 5-10% * M6 (Acute erythroid leukemia): <5% * M7 (Acute megakaryocytic leukemia): 3-10%
Some very rare variants of AML are not included on this list. Variants of AML from M3 to M7, which have their own names and some specific features, are discussed in more detail in separate sections of our reference book.
Frequency of occurrence, risk factors
AML accounts for about 15% of all cases of cancer of the hematopoietic system in children, that is, it occurs much less frequently in them than acute lymphoblastic leukemia. Among children under 14 years of age, the incidence of AML is approximately 0.6-0.8 cases per 100 thousand people per year, but after the age of 40-45 years there is a sharp rise in incidence. Most patients with AML are elderly. Unlike acute lymphoblastic leukemia, where the peak incidence is observed in childhood, with AML children make up only 10% of patients.
In most cases of AML, it is impossible to name the direct cause of the disease. However, some factors increase the likelihood of developing AML: exposure to a number of chemicals, ionizing radiation (including during previous treatment of other cancers). Sometimes cases of AML are observed among close relatives, which indicates a certain role of genetic predisposition. But specific genetic features that increase the risk of developing leukemia cannot yet be identified in many cases, although research is constantly being conducted.
The development of AML may be preceded by certain diseases of the hematopoietic system, such as myelodysplastic syndrome. Then they talk about secondary leukemia. Finally, the risk of AML is increased with certain genetically determined anomalies, including Down syndrome, Fanconi anemia, and others.
Signs and symptoms
AML is characterized by many different features and may present differently in different patients. The leading signs, as a rule, are anemia: fatigue, pallor, shortness of breath, loss of appetite. A lack of platelets is manifested by increased bleeding from cuts and bruises, nosebleeds, and the “unreasonable” appearance of bruises and hemorrhages. Treatment-resistant infections often occur because the patient has too few “normal” (mature) white blood cells to fight them. Damage to the mucous membranes of the mouth and gastrointestinal tract and swelling of the gums may occur. Body temperature is often elevated and bone pain is felt. Sometimes tumors arise from leukemic cells outside the bone marrow - myelosarcoma.
Since most symptoms can be associated with other diseases and are not specific to AML, before starting treatment it is necessary to clarify the diagnosis based on laboratory methods, which is urgently carried out in a hospital setting.
Diagnostics
With AML, changes occur in the usual clinical blood test: a lack of red blood cells and platelets, often an excess of white blood cells, many of them represented by immature forms. But a reliable diagnosis can only be made by examining a bone marrow sample. According to the World Health Organization criteria, the diagnosis of AML is made if the myeloblast content in the bone marrow is at least 20% (according to the French-American-British FAB classification, the threshold value is 30%).
To treat and assess the prognosis of the disease, it is important not only to confirm the diagnosis of leukemia, but also to distinguish between acute lymphoblastic leukemia and AML, to distinguish between leukemia and myelodysplastic syndrome, and also to determine the specific variant of AML (see above). For this purpose, not only morphological examination of cells is used (microscopic examination of specially stained preparations), but also cytochemical analysis, as well as immunophenotyping (study of antigen proteins on the surface of cells). Cytogenetic studies are used to detect chromosomal rearrangements - primarily translocations, which are of great importance for determining the variant of the disease and prognosis. Molecular genetic analyzes are also playing an increasing role.
Accurate diagnosis of AML and its variants is sometimes challenging, requiring the participation of highly qualified hematologists and hemopathologists in the diagnostic process.
As with acute lymphoblastic leukemia, the results of diagnostic tests for AML are used to assign a patient to a particular risk group. This is necessary in order to determine treatment tactics and assess both the likelihood of response to chemotherapy and the risk of subsequent relapse. Patients in the low-risk group are more likely to achieve long-term remission with standard chemotherapy treatment, while those at high risk will be considered for bone marrow transplantation to ensure the success of therapy.
The risk group is determined by many factors. Let's list some of them:
- Age: Middle-aged and elderly patients have a worse prognosis on average than children and young adults.
- Chromosomal changes in leukemic cells. Thus, translocations t(15;17) or t(8;21) determine a lower risk in patients. At the same time, for example, certain cytogenetic changes are associated with a worse prognosis.
- Variant of leukemia. Some variants of AML (such as M0, M6, M7) are associated with a high risk, and some, on the contrary, respond relatively well to modern therapy (AML M3).
- A higher risk is associated with secondary leukemia that occurs in the context of another hematological disorder, such as myelodysplastic syndrome or Fanconi anemia, or after treatment (chemotherapy, radiation) for a malignant tumor. The risk also increases when leukemia relapses.
Treatment
The main treatment for AML is chemotherapy. As in the case of acute lymphoblastic leukemia, treatment includes remission induction and consolidation phases; Sometimes maintenance therapy is also used.
Remission induction is intensive therapy aimed at achieving remission of leukemia. In most types of AML, induction of remission is carried out using intensive chemotherapy using cytarabine (Cytosar) and anthracycline drugs (daunorubicin, idarubicin), sometimes with the addition of other drugs - for example, etoposide or mitoxantrone.
A specific feature of therapy for acute promyelocytic leukemia (AML M3) is the use of the drug ATRA (all-trans retinoic acid) or other drugs with a similar effect (arsenic trioxide).
If, as a result of induction therapy, it turns out that there are less than 5% of blast cells in the bone marrow (against the background of restoration of hematopoiesis) and the patient has no other manifestations of the disease, then remission is stated.
As a result of induction courses, remission can be achieved in most patients. However, the achieved remission cannot be stable without consolidation therapy, that is, consolidation of remission. In the consolidation phase, residual amounts of abnormal blast cells are destroyed to avoid relapse of the disease. The most important drug in the consolidation phase of the treatment of AML is cytarabine, including high-dose; other drugs are often added to it in different combinations.
Chemotherapy regimens that include fludarabine are often used in the treatment of relapsed AML.
During the induction and consolidation stages, intravenous administration of chemotherapy drugs is performed in a hospital setting.
Maintenance therapy is not used in all cases (unlike acute lymphoblastic leukemia), but in the M3 AML variant it is important. This therapy is less intensive than induction and consolidation therapy and does not require a hospital stay.
Sometimes modern targeted drugs are added to therapy for AML. Specific medications depend on the genetic makeup of the tumor cells. For example, for a certain genetic disorder, the drug sorafenib is effective.
Neuroleukemia is less common in AML than in acute lymphoblastic leukemia. But sometimes it occurs, more often in children with AML variants M3, M4 and M5. For its treatment and prevention, cytarabine, methotrexate and glucocorticosteroids administered intrathecally through lumbar puncture of the spinal canal can be used.
To reduce the likelihood of relapse in some patients, bone marrow transplantation is indicated. It is used more often in AML than in acute lymphoblastic leukemia. Indications for transplantation may include, for example,
- a disease variant associated with a higher risk (see above),
- cytogenetic changes in leukemic cells associated with high risk,
- relapse of leukemia,
- secondary AML, which developed against the background of myelodysplastic syndrome or other blood disease, or after previous treatment of malignant tumors.
The likelihood of transplant success is greatest if it is performed after the first remission has been achieved. Transplantations are performed more frequently in children than in adults and are, on average, more successful. Work is constantly underway to improve transplantation regimens.
During intensive chemotherapy for AML, normal hematopoiesis is almost always suppressed to some extent. Therefore, many AML patients require transfusions of blood components: platelets to prevent bleeding and red blood cells to treat anemia.
Since both leukemia itself and the chemotherapy used in its treatment sharply reduce the body’s resistance to various infections, patients during treatment often need effective antibacterial, antifungal and antiviral drugs for the prevention and treatment of infectious complications. Both common and opportunistic infections pose a danger.
Treatment of AML imposes restrictions on the patient's lifestyle. During intensive chemotherapy, it is necessary to follow a diet and strict hygiene rules, as well as minimize contact with the outside world to avoid infections. Doctors and nurses tell each patient what he can and cannot do at the current stage of treatment.
The total duration of treatment for various forms of AML ranges from several months to (in the case of variant M3) 2-3 years.
New targeted drugs are constantly emerging to help cure patients with complex cases of AML. Thus, some patients benefit from venetoclax (Venclexta) and/or daratumumab (Darzalex). The conjugate drug gemtuzumab ozogamicin (Mylotarg) is sometimes used. In addition, bone marrow transplantation protocols are being improved.
Forecast
Without treatment, AML usually leads to death of the patient within several months, sometimes even several weeks. However, with modern treatment many can be saved. The prognosis depends on the specific type of leukemia, chromosomal characteristics of leukemic cells, age, general condition of the patient and other factors.
Currently, medicine in developed countries is able to cure more than 65% of children with AML. In particular, the results of bone marrow transplantation are constantly improving. In middle and old age, unfortunately, the likelihood of cure is significantly lower.
It should be noted that the majority of patients with AML are elderly patients. Compared to young people, they tolerate treatment more difficultly and respond less well to it. Only a minority of patients over 60 years of age achieve long-term remission. For others, supportive treatment (fighting infections, transfusions of blood components, pain relief) is often the mainstay to prolong life and improve its quality.