Approximately 75% of boys born with this anomaly experience spontaneous migration of the testicles within 6 months, and only 1–2% of newborns still have cryptorchidism by the end of the first year of life.
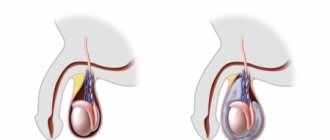
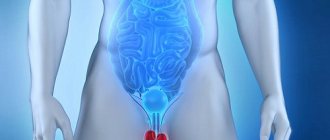
A congenital defect of the left testicle is observed in 20% of cases, the right - in 50%. Bilateral cryptorchidism is diagnosed in 30% of children. Pathology is divided into two groups: ectopia and testicular retention. In the first case, the testicle is fixed in the groin, penis or thigh area, in the second, the testicle stops in the abdominal cavity or inguinal canal on its way to the scrotum. In both cases, it is not possible to manually lower the testicle into the scrotum.
If the testicle has not migrated into the scrotum due to tension in the levator muscle, this anomaly is classified as false cryptorchidism.
, which does not require surgical intervention, disappearing on its own during puberty. There is also a type of disease called an ascended testicle, in which it moves upward, which is caused by too slow growth of the spermatic cord. In any case, before prescribing treatment, a thorough diagnosis of cryptorchidism is carried out to determine the type of pathology.
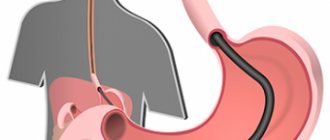
Right-sided cryptorchidism
Causes
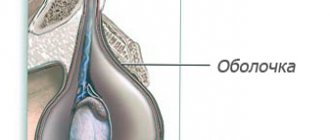
To penetrate the scrotum during the intrauterine stage of fetal development, the testicle makes its way through the abdominal cavity and passes through the abdominal wall along the inguinal canal. In the embryonic period, the testicle is located first retroperitoneally, and then intraperitoneally. During the first three months of intrauterine life, the gonad is located in the lumbar region, on the side of the spine, adjacent to the kidney. By the end of the 3rd month of intrauterine life, the gonad is located near the internal opening of the inguinal canal. Only at the 8th month does the testicle penetrate the inguinal canal and from here descend into the scrotum. The gonad descends into the scrotum following the guide ligament - gubernaculum testit. The canal through which the testicle enters the scrotum is represented by the vaginal process of the peritoneum, which is obliterated (closed) by the time the baby is born.
The process of lowering the testicle into the scrotum occurs under the influence of several main factors:
- downward traction due to the gonad conductor - Gunter's cord;
- difference in the growth rate of the body and the growth of the spermatic cord;
- increased intra-abdominal pressure;
- bowel movements, the lower section of which, during the final descent of the testicles, is filled with meconium;
- appendage development.
The passage of the testicle through the inguinal canal largely depends on the concentration of androgens (male sex hormones) produced by the embryonic gonad. The greatest role is given to luteinizing hormone, which is actively produced by the pituitary gland of the fetus in the last trimester of pregnancy. Thus, a decrease in the concentration of male sex hormones that are produced in the fetus or a deficiency of gonadotropic hormones in the mother (it has been established that gonadotropic hormones affect the descent of the testicles by regulating the production of androgens) can significantly affect the process of testicular descent. Any mechanical obstacles along the route can also lead to its deviation.
Risk factors
Experts identify the following reasons that may increase the risk of developing an undescended testicle:
- underweight newborn;
- premature birth;
- hereditary factor associated with an undescended testicle or a number of other pathologies of genital development;
- Down syndrome, abdominal wall defect, and other conditions associated with normal fetal development;
- drinking alcohol, drugs, smoking and second-hand smoke during pregnancy;
- overweight or obesity in a woman in labor;
- the presence of diabetes mellitus in the woman in labor: diabetes mellitus types 1 and 2, as well as gestational diabetes;
- exposure to certain pesticides.
Most common complications
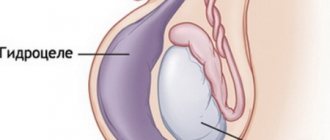
- Dropsy of the membranes of a retained testicle with cryptorchidism. Hydrocele of the testicular membranes can occur when the processus vaginalis of the peritoneum is not closed;
- Strangulated hernia;
- Testicular torsion;
- The risk of developing testicular cancer in patients with cryptorchidism is 10 times higher than in men in the general population. Of all diagnosed seminomas, 50% are detected in undescended testicles, especially often with intra-abdominal location of the testicle. In addition to seminomas, men with cryptorchidism have a high incidence of chorionepitheliomas and teratoblastomas;
- Infertility. The greatest risk of developing infertility (70%) is in patients with bilateral cryptorchidism. Right- or left-sided is not so dangerous, since one testicle with cryptorchidism retains functionality. Long-term results of surgical treatment show that infertility develops in 50-60% of patients operated on for the disease over the age of 5 years.
Classification of cryptorchidism
Cryptorchidism is divided into retention and ectopia.
- Retention is a condition in which one or both testicles do not descend into the scrotum, but are delayed somewhere along the way from the place of their formation (at the lower pole of the kidneys) to the external opening of the inguinal canal.
- With cryptorchidism, testicular retention can be: 1) in some place in the intraperitoneal part of the path of its descent (to the internal opening of the inguinal canal) - intraperitoneal (retention testis abdominalis); 2) in the inguinal canal at its various levels - inguinal (retention testis inguinalis);
- Ectopia is a condition in which the testicle has deviated somewhere from its path into the scrotum. With this pathology, the testicular conductor is of great importance, which is not attached to the bottom of the scrotum, as it should be normally, but directs the gonad away from the scrotum. The spermatic cord with ectopic testicle is of normal length.
There are five types of ectopia: 1) superficial ectopia - the most common type of ectopia. The testicle is located under the skin of the abdomen above the aponeurosis of the external oblique muscle; 2) penis-pubic ectopia - the gonad is located at the base of the penis and lies on the pubic bone; 3) femoral ectopia - located in Scarp’s triangle, closer to the medial surface of the thigh; 4) transverse ectopia - the testicles pass through the same inguinal canal and are located in one half of the scrotum; 5) perineal ectopia - the gonad is located in the perineal area.
A rare type of cryptorchidism is observed when the testicle and epididymis exist separately at a greater or lesser distance from each other, being connected only by a connective tissue cord.
The role of the scrotum for thermoregulation and spermatogenic function of the testicle
The scrotum is an important thermoregulatory structure for the gonads. Lower temperature in the scrotum is an important factor ensuring normal spermatogenesis. When the testicle is located in the inguinal canal and, especially in the abdominal cavity, its function is impaired, since under these conditions the optimal temperature, which is extremely necessary for development and mainly for normal spermatogenesis, is not provided. The temperature in the abdominal cavity and inguinal canal is higher than in the scrotum. In children, this difference reaches 2-5 degrees.
Due to temperature differences, the formation of bilateral cryptorchidism often leads to infertility.
Diagnosis of cryptorchidism
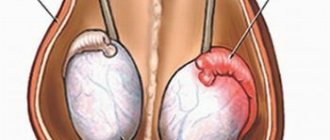
Cryptorchidism is easily diagnosed. Upon examination, you may notice the absence of one or both gonads in the scrotum and swelling in the groin when the testicle is retained in this area. It is not possible to manually lower the testicle into the scrotum. With both bilateral and unilateral cryptorchidism, the scrotum is underdeveloped to a greater or lesser extent.
Patients with proximal forms of hypospadias, micropenia, or a non-palpable testicle often require a differentiated approach to treatment. Such children need consultation with an endocrinologist, geneticist, and additional examination methods to determine the karyotype in order to exclude pathology of sex formation.
Scheduling a doctor's visit
Typically, the problem is discovered soon after birth, and your doctor or pediatrician should monitor the scrotum regularly. Before you meet with your doctor, write down a list of questions to discuss. If you have additional questions for a specialist, do not hesitate to ask them. — How often should you schedule visits to the doctor? — How to examine a child’s scrotum at home? — What diagnostics does the child need? — What treatment options are recommended to eliminate the pathology?
What is false cryptorchidism?
A false form of cryptorchidism or an increased cremasteric reflex is a normal condition in which the cremasteric muscle pulls the testicle into the inguinal canal. The formation of false cryptorchidism is observed in children under stress and freezing. Most often, an increased cremasteric reflex is observed at the age of 6-7 years, when the false form of the disease is most often detected. In this case, the gonad can be manually lowered to the bottom of the scrotum and it remains there for some time; in a warm bath, the testicle descends into the scrotum on its own, which indicates a false disease. The false form of cryptorchidism does not require treatment.
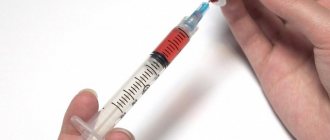
Cryptorchidism: Drug treatment
Drug treatment is carried out with chorionic gonadotropin preparations. The success rate of conservative treatment is about 20 percent. Moreover, 20% of patients experience a relapse after discontinuation of conservative treatment. The higher the gonad is initially located, the lower the effectiveness of hormonal therapy.
There are different dosage regimens and frequency of administration of human chorionic gonadotropin during treatment, but there are no significant differences in the results of using different treatment regimens. The standard regimen for administering human chorionic gonadotropin drugs is: injections 2 times a week for 4 weeks intramuscularly. If cryptorchidism is present, treatment should begin after reaching 6 months of age. Luteinizing hormone-releasing hormone (LHRH) analogues, administered in pulse mode, are also used to treat cryptorchidism. The effectiveness of this therapy does not differ from the effectiveness of treatment with human chorionic gonadotropin.
Surgical treatment of cryptorchidism (more details in the surgical treatment section)
Essentially, testicular reduction is a plastic operation of moving the organ and forming new connections and relationships (Gusynin V.O., 1937). The main goal of surgical treatment of cryptorchidism is the mobilization and lengthening of the spermatic cord with the release of the testicle, the formation of a bed for the testicle in the scrotum, its reduction and fixation in the proper place without tension on the vessels, which allows the normal development of the reduced testicle.
Cryptorchidism (abdominal retention, inguinal retention, high testicle)
Cryptorchidism (from the Greek kryptos - hidden and orchis - testicle) is a congenital malformation in which one or both testicles have not descended into the scrotum at the time of birth.
EPIDEMIOLOGY
The urgency of the problem is due to the high frequency of infertile marriages in patients with various forms of cryptorchidism, which is 15-60%. According to various authors, cryptorchidism occurs in newborn full-term boys in 3% of cases, in premature infants - up to 30% of cases.
According to the literature, right-sided cryptorchidism occurs in 50% of cases, bilateral - in 30%, and left-sided - in 20% of cases.
ETIOLOGY AND PATHOGENESIS
The process of testicular descent is a completely unexplored aspect of sexual differentiation, both with respect to the nature of the forces causing testicular movement and the hormonal factors that regulate this process.
It is customary to distinguish five stages of testicular migration:
- laying of the gonad;
— migration of the testicle from the place of formation of the gonad to the entrance to the inguinal canal;
- formation of an opening in the inguinal canal (vaginal process), through which the testicle leaves the abdominal cavity;
- passage of the testicles through the inguinal canal into the scrotum;
- obliteration of the vaginal process of the peritoneum.
The process of migration of the testicles from the abdominal cavity to the scrotum begins from the 6th week of intrauterine development of the fetus. The testicles reach the inner ring of the inguinal canal at approximately 18-20 weeks, and by the time the fetus is born, the gonads are located at the bottom of the scrotum. If the transabdominal path of testicular migration does not depend on the level of androgens, and is possibly mediated by intra-abdominal pressure and the paracrine influence of growth peptides of local or testicular origin, then the passage of the testicle through the inguinal canal is sufficiently dependent on the concentration of androgens produced by the embryonic testicle. However, the leading role at this stage belongs to LH, actively produced by the fetal pituitary gland in the last trimester of pregnancy.
Many congenital anomalies associated with a defect in testosterone biosynthesis, dysfunction of Sertoli cells secreting anti-Mullerian factor, insufficiency of gonadotropin production, are accompanied by cryptorchidism (Kalman, Klinefelter, Prader-Willi, Noonan syndromes, etc.). In addition, cryptorchidism is one of the symptoms of genetic disorders that cause multiple developmental anomalies (Carnelius de Lange, Smith-Lepley-Opitz syndromes, etc.). However, in some patients with cryptorchidism, primary disorders of gonadotropic and gonadal functions are not detected, especially in its unilateral form. Apparently, cryptorchidism is a consequence of multifactorial disorders in which hormonal deficiency does not always play a major role. The leading role in the development of cryptorchidism is probably played by genetic disorders leading to a lack of paracrine factors produced by both testicles and vascular cells, the vas deferens, and the inguinal canal.
The main consequence of cryptorchidism is a violation of the germinal function of the testicle. Histological examination of the testicles reveals a decrease in the diameter of the seminal ducts, a decrease in the number of spermatogonia and foci of interstitial fibrosis. Similar abnormalities in undescended testicles were detected in 90% of children over 3 years of age. There is information in the literature about structural changes in Leydig and Sertoli cells in cryptorchidism in older boys. It remains a matter of debate whether these changes will be a consequence of cryptorchidism or its cause. There are opinions that changes in the testicle with cryptorchidism are primary. This is confirmed by the fact that in patients with undescended testicles, pathological changes in the tubular epithelium do not occur with age. Impaired fertility, even with timely lowering of the testicles, is noted in 50% of patients with bilateral and 20% of patients with unilateral cryptorchidism.
The risk of developing testicular neoplasia in patients with cryptorchidism is 4-10 times higher than in men in the general population. Of the total number of testicular seminomas diagnosed, 50% are found in undescended testicles. Testicles located in the abdominal cavity undergo malignancy more intensively (30%) than, for example, those located in the inguinal canal. Reduction of the testicle does not reduce the risk of malignancy, but allows for timely diagnosis of the neoplasm. In 20% of cases, tumors in patients with unilateral cryptorchidism develop in the contralateral testis. In addition to seminomas, men with cryptorchidism have a high incidence of gonocytes and carcinomas. The fact that this type of tumor develops may also support the theory of primary dysgenesis of the undescended testicle.
Currently, most researchers propose dividing patients with cryptorchidism into two groups. The first group includes patients with a short spermatic cord. The main causes of the disease include genetic, hormonal, receptor and paracrine causes. The second category includes patients with various forms of ectopia of the male gonad (inguinal, perineal, femoral, pubic and heterolateral), which are based on the mechanical theory of impaired testicular migration.
The division into groups of different pathogenesis is due to a fundamentally different approach to the treatment tactics of patients with this disease. In the first group, where the problem is initiated by testicular retention (delay of the gonad on the way to migrate to the scrotum), preoperative preparation with the use of gonadotropins is necessary. The goal of hormonal therapy is to lengthen the vascular bundle of the male gonad, which allows the testicle to be brought down into the scrotum with minimal tension. Tension of the vascular bundle leads to a decrease in the diameter of the vessels supplying the gonad and, accordingly, to a deterioration in the trophism of the organ. The vessels that supply the walls of the main vessels of the spermatic cord also suffer, causing swelling of the vessel wall, reducing its diameter, which again negatively affects blood flow, contributing to ischemia of the testicular tissue.
Currently, the negative effect of short-term ischemia on testicular tissue has been proven. Already after three hours of gonadal ischemia, with torsion of the spermatic cord, diffuse necrosis occurs in the testicular tissue. After 6-8 hours from the moment of torsion, almost the entire gonad undergoes necrosis.
Thus, one of the most important tasks facing the surgeon is mini-
mitigation of testicular tissue ischemia during surgical correction of cryptorchidism.
Accordingly, the entire arsenal of known operational aids should be
used taking into account the pathogenesis of secondary infertility associated with disorders
trophism of the gonads.
CLINICAL PICTURE
When examining a patient with a presumptive diagnosis of cryptorchidism, it must be remembered that in some cases it is possible to identify children with false cryptorchidism or with an increased cremasteric reflex. In such children, the scrotum is usually well developed. When palpating in the groin area, in the direction from the inner ring of the inguinal canal to the outer ring, the gonad can be brought down into the scrotum. Parents of such a child often note that while swimming in warm water, the testicles spontaneously descend into the scrotum.
In children with true cryptorchidism, the testicle cannot descend into the scrotum. In this case, one or both halves of the scrotum are hypoplastic, and the gonad is palpated in the inguinal, femoral, pubic, perineal region or in the opposite half of the scrotum. Of particular interest is a testicle palpated in the groin area, since in this case there is a need for differential diagnosis of inguinal ectopia of the gonad with inguinal retention. With any form of gonadal ectopia, there is practically no need for hormonal preoperative preparation, since the elements of the spermatic cord are well defined and have sufficient length for free surgical reduction into the scrotum.
However, with inguinal retention, the gonad is located in the inguinal canal, and the testicular vessels do not have sufficient length for free retraction. This is why patients with inguinal gonadal retention require preoperative hormonal therapy.
Unfortunately, it should be noted that hormonal therapy is not always successful. According to one version, the cause may be a blockade of androgen receptors in testicular vessels, which can be complete or partial. Perhaps this can explain the effectiveness of hormonal therapy for a certain group of patients, the insignificant effect in patients with partial blockade of receptors and the complete lack of dynamics in patients with complete blockade. It should be noted that hormonal therapy is least likely to be effective in patients whose testicles are located in the abdominal cavity. Presumably, the degree of dysgenesis and receptor activity directly depend on the severity of the pathological process.
It is often possible to differentiate inguinal ectopia from inguinal retention by performing a palpation examination. In cases where the gonad palpated in the groin area moves exclusively along the canal, repeating its anatomical course, i.e. limited by the walls of the inguinal canal, testicular retention can be stated with a high degree of certainty. And, on the contrary, the displacement of the gonad in almost all directions indicates inguinal ectopia.
The most difficult group is patients with abdominal retention, both from a diagnostic and treatment point of view. First of all, in a patient with non-palpable testicle syndrome, it is necessary to determine the gender, excluding chromosomal sex disorders. In this case, first of all, differential diagnosis must be carried out with mixed gonadal dysgenesis.
Mixed gonadal dysgenesis is a condition in which phenotypic men or women have a testicle on one side, and a fallopian tube, a connective tissue cord, and sometimes a rudimentary uterus on the other. A cord (strek) is a thin, pale, elongated formation, often oval in shape, located either in the broad ligament or on the pelvic wall, consisting of ovarian stroma.
Karyotyping reveals 45XO/46XY mosaicism in 60% of patients with this anomaly, and 46XY mosaicism in 40% of male patients. Most often, the genitals of a patient with this anomaly have a bisexual structure. In cases where the male phenotype is dominant, patients are diagnosed with one of the forms of hypospadias and, as a rule, infertility.
In such cases, the patient is assigned a female gender and feminizing operations are performed with the removal of rudimentary internal genital organs. Much less often, usually for social reasons, the gender is left male. For this purpose, laparoscopic removal of the uterus, fallopian tube and streca is performed, and the testicle is either removed, transferring the child to hormone replacement therapy in the future, or relegated to the scrotum, and the child’s parents are warned about the high probability of malignancy of the gonad, the frequency of which is in patients with mixed gonadal dysgenesis reaches 20-30%.
Algorithm for examining patients with non-palpable testicle syndrome
includes an ultrasound scan of the abdominal cavity, however this method
diagnostics, unfortunately, are not always reliable.
Modern high medical technologies make it possible to use radioisotope methods, angiography, CT, MRI, etc. to diagnose severe forms of cryptorchidism. However, laparoscopic examination is the most objective and reliable method for diagnosing this disease at present. It allows you to assess the condition of the gonad vessels, accurately determine the location of the testicle and assess the condition of the gonad based on external signs. In case of severe testicular dysplasia, an orchidectomy is performed. In doubtful cases, a gonadal biopsy is performed.
Hormonal treatment using gonadotropins does not always achieve the desired result, but in some patients it is still possible to lengthen the testicular vessels. The defining sign of the effectiveness of the therapy is the displacement of the gonad to the opposite ring of the inguinal canal during repeated diagnostic laparoscopy.
Repeated laparoscopy is performed 1-3 weeks after the course of hormonal treatment. In cases where it is possible to achieve a positive effect to a greater or lesser extent, immediately after assessing the length of the gonad vessels, they switch to the open method of surgical reduction of the testicle.
TREATMENT
DRUG TREATMENT
Treatment of cryptorchidism is carried out with chorionic gonadotropin preparations. Despite the fact that hormonal therapy for cryptorchidism has been widely used for more than 30 years, information about its effectiveness is extremely contradictory. From the point of view of endocrinologists, the effectiveness of hormonal therapy is due to the group of patients where the testicles were previously located in the scrotum. When treating true cryptorchidism, the effectiveness does not exceed 5-10%. Efficiency refers to the movement of the gonad into the scrotum under the influence of hormonal therapy, but the length of the testicular vessels is not assessed.
There are different dosage regimens and frequency of administration of human chorionic gonadotropin in the treatment of cryptorchidism, but there are no significant differences in the results of using different treatment regimens. The standard regimen for administering human chorionic gonadotropin drugs is: injections 2 times a week for 5 weeks intramuscularly. Treatment should begin after the child reaches one year, using the following doses of human chorionic gonadotropin: 1.5-2 years - 300 units per injection; 2.5-6 years - 500 units; 7-12 years - 1000 units. For the treatment of cryptorchidism, analogues of luteinizing hormone releasing hormone (LHRH), prescribed in a pulse mode, are also used. The effectiveness of this treatment does not differ from the effectiveness of treatment with human chorionic gonadotropin.
SURGICAL TREATMENT
Despite extensive clinical experience in the surgical treatment of cryptorchidism throughout the world, there is no uniform concept on the timing of intervention. Most clinicians recommend starting treatment as early as possible: W. Issendorf and S. Hofman (1975), R. Petit and Jennen (1976), S. Waaler (1976) - at 5 years; A.G. Pugachev and AM Feldman (1979) - at 3 years old; N.L. Kushch (1979) - at 2 years old; T.V. Semecheva, A.N. Tyulpakov, A.P. Erokhin, SI. Volozhin, A.K. Fayzulin, Berku, Donahoe, Hadziselimovic (2007) - in the 1st year; S. Hecker (1977) - on the 4-5th day of life.
Long-term results of surgical treatment show that infertility develops in 50-60% of patients operated on for cryptorchidism over the age of 5 years. In the era of conservative treatment of cryptorchidism using hormonal therapy, there was an opinion that this treatment was quite effective without surgical correction. However, in 90% of cases, cryptorchidism is not accompanied by fusion of the vaginal process of the peritoneum. In such patients, after migration of the testicle into the scrotum, it is necessary to perform operations to prevent the development of inguinal hernia and testicular hydrocele.
Clinicians often encounter a situation where, several months after hormonal therapy, the gonad is again pulled up to the level of the inguinal canal. This circumstance once again demonstrates the need for surgical intervention to ligate the vaginal process of the peritoneum and perform orchiopexy. All known surgical methods for the treatment of cryptorchidism are divided into two groups: one-stage and two-stage. One-stage methods include operations that allow you to isolate and ligate the vaginal process of the peritoneum at the internal ring of the inguinal canal, mobilize elements of the spermatic cord, bring the testicle into the scrotum and perform temporary or permanent fixation of the gonad. Two-stage methods, in turn, can also be divided into two subgroups:
— operations performed with a moderate deficiency in the length of the gonad vessels (fixation of the testicle to the lata fascia of the thigh with the imposition of a femoral-scrotal anastomosis);
— operations performed when there is a pronounced deficiency in the length of the gonad vessels, when the testicle cannot be lowered into the scrotum.
The first operation for cryptorchidism was performed by Koch from Munich in 1820. On the advice of CheliusoH, he opened the scrotum, passed a ligature through the vaginal membrane and applied a pelot in the hope that subsequent traction on the ligature would bring the testicle down into the scrotum. This operation ended in the death of the patient as a result of developing peritonitis. The first successful testicular reduction operation was performed in 1879 by Annandale on a three-year-old boy with perineal ectopia on the right. Annandale sutured the testicle to the bottom of the scrotum with a subcutaneous catgut suture.
Of the most common treatment methods, the first group includes the methods of Petriwalasky (1932), Schoemaker (1931), Ombredanne (1910), Welch (1972), Perrone, Signorelli (1963). Recently, the most widely used method is the Schoemaker-Petriwalasky method, which makes it possible to optimally lower the gonad into the scrotum and fix it in a subcutaneous pocket at the bottom of the scrotum.
The idea of Ombredanne, Welch, Perrone, Signorelli, which is based on fixation of the reduced gonad to the interscrotal septum, remains interesting. The methods differ only in the relationship of the gonad to the septum. The disadvantage of the method is the inability to perform this intervention as a result of a pronounced deficiency in the length of the spermatic cord.
The fundamental advantage of these technologies is the direct direction of the testicular vascular bundle without artificially created kinks. This technique allows you to minimize the degree of gonadal ischemia caused by kinking of the spermatic cord.
The first subgroup of two-stage technologies includes the Keatley-Bayle-Thorek-Herzen method. The first stage of the method is based on ligation of the vaginal process of the peritoneum, mobilization of the vascular bundle and fixation of the gonad to the broad ligament of the thigh with the creation of a femoral-scrotal anastomosis. Three months later, the femoral-scrotal anastomosis is divided, the gonad is isolated and cut off from the broad ligament with immersion into the scrotum. Disadvantages of the method:
— cases with a pronounced deficiency in the length of the spermatic cord, when this technology is not feasible;
- bending of the spermatic cord at the level of the outer ring of the inguinal canal (may contribute to hemodynamic disturbances in the gonad)
— a scar process that occurs perifocally in the area of testicular implantation, with a high degree of probability leads to irreversible changes in the gonad.
The second subgroup includes operations in which a pronounced deficiency in the length of the spermatic cord does not allow the gonad to be brought down into the scrotum. In these cases, step-by-step reduction is performed. During the first stage, the vaginal process of the peritoneum is treated and the testicle is fixed at the point of maximum reduction. Subsequently, after 3-6 months. after the first stage of surgical treatment, the gonad is isolated from the surrounding tissues and brought down into the scrotum. The disadvantage of the method is a pronounced scar process that forms around the reduced gonad after the first stage of surgical treatment, which can negatively affect the function of the organ in the future.
The “long loop duct” operation developed and implemented by R. Fowler and FD Stephens in 1963 should also be included in this group. The principle of the operation is to intersect the vessels of the testicle while preserving the collateral branches and vessels of the vas deferens.
The frequency of decreased fertility in patients with cryptorchidism does not always depend on the degree of gonadal dysgenesis. Often the cause of infertility can be a pathogenetically unsubstantiated method of surgical correction of this disease, leading to ischemia of testicular tissue.
X Operations using the principle of temporary fixation of the testicle include the method developed by Mixter (1924). The operation begins from the same incision as for hernia repair. The aponeurosis of the external oblique muscle is exposed layer by layer. The anterior wall of the inguinal canal is dissected and its revision is carried out. Most often, the testicle is located along the inguinal canal or at its outer ring. In some cases, with inguinal retention of the testicle, it can wander, being either in the abdominal cavity or in the inguinal canal. That is why it is not always possible to palpate the gonad in the inguinal canal. In cases where the testicle is located in the abdominal cavity, it is first brought out, then the hernial sac is isolated.
When using microsurgical instruments and optical magnification, it is optimal to isolate the vaginal process using the open method. It is possible to use hydropreparation of tissues. The isolated hernial sac is stitched and bandaged at the internal ring of the inguinal canal, after which they begin to mobilize the elements of the spermatic cord.
An important point in the operation of testicular reduction is the maximum isolation of the elements of the spermatic cord with dissection of the fibrous cords accompanying the vessels, which allows to significantly increase the length of the neurovascular bundle. If necessary, mobilization is performed retroperitoneally until the testicle reaches the scrotum. Sometimes, despite preoperative hormonal preparation, the testicular vessels still remain short. In this situation, dissection of the lower epigastric vessels is performed. This intervention option was proposed by Prentiss (1995). The principle of this manipulation is to reduce the distance from the beginning of the testicular vessels to the scrotum by reducing the angle in the seminal surgical triangle. The testicle can also be passed through a shorter route, preserving the epigastric vessels. For this purpose, a curved Billroth-type clamp is used to bluntly create a hole in the posterior wall of the inguinal canal. The clamp is passed under the epigastric vessels, grabbed by the membranes or by the remains of the Gunther's cord and passed through the newly formed opening in the posterior wall of the inguinal canal.
The principle of fixing a descended testicle in the scrotum according to Mixter is to apply a pierced ligature, brought out through the skin of the scrotum and fixed to the skin of the thigh. A fixing ligature is placed in the area of transition of the tunica albuginea into the testicular tunica propria, at the lower pole. The choice of the distal fixation point is determined by preliminary “trying on” in order to prevent pronounced tension on the elements of the spermatic cord. Then the inguinal canal is sutured from top to bottom. The outer ring of the inguinal canal should not compress the elements of the spermatic cord. For this purpose, the last suture on the anterior wall of the inguinal canal is applied under the control of the fingertip. The wound is sutured tightly in layers. The fixing ligature and skin sutures are removed on the 7th day after surgery.
The Keetley-Torek operation differs from this technology by fixing the testicle to the fascia lata of the thigh by creating a femoral-scrotal anastomosis. After processing the vaginal process of the peritoneum and mobilizing the gonad, a ligature-leash is applied to the remains of the Gunther's cord. The scrotum is incised at the lowest point, making an incision 2-3 cm long. A Billroth type clamp is passed through the incision, a ligature is grasped and the testicle is brought out. Using the “trying on” method, the level of fixation of the gonad to the inner surface of the thigh is determined. A transverse incision is then made on the thigh, similar to the incision on the scrotum.
According to the Keetley technology, the testicle is not removed from the scrotum, but is fixed with separate sutures to the remnants of the Gunther's cord to the lata fascia of the thigh. The edges of the scrotal skin incision are sutured to the edges of the thigh skin incision, forming a femoral-scrotal anastomosis. According to the Torek method, a bed for the testicle is created on the scrotum and then the gonad is fixed to the fascia lata of the thigh, after which a femoral-scrotal anastomosis is performed. The wound in the groin area is sutured using the method described above.
After 6-8 weeks, a division of the anastomosis is performed, the testicle is immersed in the scrotum.
The Fowler method (1972) is considered one of the attempts to abandon methods of rigid fixation of the gonad to the thigh. The principle of the operation is to pass a fixing ligature through the lower part of the scrotum and apply a perineal suture behind the scrotum so that when tying there is no pronounced traction on the testicular vessels. When fixing according to Fowler, the testicle is always slightly retracted to the posterior surface of the scrotum, without giving a characteristic protrusion of its contours. The fixing ligature and skin sutures are removed on the 7th day.
The principle of fixation of the gonad according to the Bevan method (1899) is that both ends of the fixation ligature are brought out through the skin of the scrotum and tied to a tube. The tube and thread are removed on the 7th day.
Passing through the fixing ligature through the skin of the scrotum is a feature of orchiopexy using the Sokolov method. The ligature is then pulled up and tied on the roller, and the ends of the thread are tied to the rubber end attached to the splint on the opposite thigh. The ligature and skin sutures are removed on the 7th day.
In cases where the operator is unable to bring the testicle into the scrotum in one stage, the principle of staged movement of the gonad is used. During the first stage, the testicle is fixed under the skin, in the pubic area, to the inguinal ligament or in the upper part of the scrotum. A prerequisite is minimal tension of the testicular vessels in order to prevent ischemia of the testicular tissue. An attempt to move the gonad into the scrotum is performed after 6-12 months.
Operations using the principle of permanent fixation. The operation of Schoemaker (1931) and Petriwalsky (1931) has become widespread throughout the world for its original method of fixing the gonad in the scrotum. Unlike many of the methods described above, this technology allows for “gentle” traction of the gonad.
The operation is performed from the inguinal approach, the inguinal canal is opened, the processus vaginalis of the peritoneum is processed and the elements of the spermatic cord are mobilized using the technology described above. The method of fixing the gonad in the scrotum is fundamentally different. For this purpose, the index finger is passed to the bottom of the scrotum, creating a tunnel through which the gonad is subsequently passed. A transverse incision about 10 mm long is made in the middle third of the scrotum at the height of the fingertip. The depth of the incision should not exceed the thickness of the skin of the scrotum itself. Then, using a mosquito-type clamp curved in the sagittal plane, a cavity is created between the skin and the fleshy shell of the scrotum. The volume of the formed cavity must correspond to the volume of the reducible gonad.
Then a “mosquito” type clamp is passed on the finger from the wound in the scrotum to the inguinal surgical wound, the membranes of the gonad are grabbed and brought out through the scrotal incision so that the hole in the fleshy membrane freely allows the elements of the spermatic cord to pass through. This technique allows you to create an additional holding mechanism for the testicle, acting as a damper with moderate tension on the gonad. The testicle is fixed with two or three sutures by the remnants of the vaginal process to the fleshy membrane.
The next step is to remove the hydatid and place the testicle in the vaginal sac, which is sutured down to the spermatic cord. The gonad is immersed in the formed bed. The skin of the scrotum is sutured with an interrupted or continuous suture. The wound in the groin area is sutured in layers. When forming the outer ring of the inguinal canal, it is necessary to remember the possible compression of the elements of the spermatic cord.
Operation Ombredanna. An incision in the groin area is used to open the anterior wall of the inguinal canal and mobilize the spermatic cord. The index finger passes through the lower corner of the wound into the scrotum and stretches the skin on the opposite side through its septum. Then the skin is incised and the scrotal septum is incised above the fingertip. Using a ligature previously sewn through the remains of the Gunther's cord, the testicle is brought out through the incision. The incision in the septum is sutured to the spermatic cord, and the testicle is immersed in the scrotum. The inguinal canal is sutured as for hernia repair. The scrotal wound is sutured tightly.
Operation Chukhrienko-Lyulko. An incision is made as for hernia repair. After mobilization of the spermatic cord, the processus vaginalis is dissected in the transverse direction. The proximal part of the process leading into the abdominal cavity is sutured with a purse-string suture and bandaged with a continuous mylar suture. Then, a superficial skin incision up to 6 cm long is made on the anterior surface of the corresponding half of the scrotum. The fleshy membrane is bluntly separated from the skin of the scrotum. An incision is made in the upper corner of the scrotum in the fleshy membrane through which the testicle is passed. The fleshy wound is sutured with Mylar sutures. Additionally, the fleshy membrane is fixed with a lavsan suture to the opposite wall of the scrotum, starting from the spermatic cord to the bottom of the scrotum. The testicle is fixed to the dense wall thus formed with the free ends of the threads with which the distal part of the processus vaginalis is sutured. The inguinal canal and the scrotal wound are sutured. As a result, the testicle becomes fixed in the lowest part of the scrotum between its skin and the double wall of the fleshy membrane.
Vermuthen's operation. The testicle bed is created not by expanding the scrotum, but by using a clamp. The threads with which the remains of the Gunter's cord are stitched are brought out through the formed bed of the scrotum using straight needles and tied. Elastic traction is established to the inner surface of the opposite thigh, as in the Gross operation, or on the side of the operation, as in Sokolov orchiopexy. The testicle is fixed in the lowest part of the scrotum between the fleshy membrane and the skin of the scrotum.
Currently, methods of bringing down the testicle and fixing it in the scrotum by suturing the spermatic cord along the inguinal canal - funiculopexy - are becoming increasingly widespread.
Reduction of the testicle into the scrotum with the formation of a new arteriovenous pedicle (testicular autotransplantation according to Kirpatovsky). It is carried out by crossing the testicular vascular pedicle, but, unlike the Fowler and Stephens method, a new vascular pedicle is formed. To do this, the vessels are connected to a new source of blood supply, which is usually the lower epigastric vessels, due to which the newly formed vascular pedicle is lengthened. This operation differs from a typical transplantation only in that the vas deferens is not crossed and vaso-vasal anastomoses are not formed, since its length is sufficient for the reduction of the testicle. Autotransplantation of the testicle on an arteriovenous pedicle is used for the most severe forms of cryptorchidism in conditions of high abdominal retention, when the testicle is located at the lower pole of the kidney on a short main vascular pedicle, or instead of a main vessel there is only an arterial network.
In this case, the operation is reduced to the intersection of the testicular artery and vein, and the vas deferens is mobilized along its entire length to the entrance to the pelvis. The testicle is removed from the abdominal cavity through an artificially created opening in the area of the medial inguinal fossa and immersed into the scrotum through the superficial opening of the inguinal canal. In the inguinal canal, the lower epigastric vessels are isolated - an artery and a vein, which are crossed, and their central ends are transposed into the inguinal canal. The blood supply in the descended testicle is restored by connecting the testicular artery and vein with the lower epigastric vessels using microsurgical techniques.
The use of microsurgical technology allows the testicle to be brought down into the scrotum by autotransplantation in cases where the insufficient length of the vascular pedicle of the testicle excludes the possibility of orchidopexy. More preferably, the testicular artery and vein are connected to the inferior epigastric artery and vein, respectively. A. Haertig et al. (1983) recommend limiting yourself to arterial anastomosis, considering venous outflow through v. to be sufficient. deferentialis. T.I. Shioshvili considers this a necessary measure, for example, in case of anomaly v. testicularis, since in this case periorchitis may develop in the postoperative period.
Van Kote (1988) believes that testicular autotransplantation is promising only in 20% of patients with abdominal cryptorchidism. The optimal age is considered to be two years, but this operation has so far been successfully performed only in two boys aged 2 years. Microsurgical autotransplantation of a testicle located in the abdominal cavity before the age of two is difficult due to the small size of the testicular vessels with a diameter of 0.4 to 0.6 mm.
In addition, it is necessary to remember the anatomical features of testicular trophism. Apparently, it is no coincidence that the testicular artery departs from the renal artery on the left and from the abdominal aorta on the right, and immediately before entering the gonad the testicular artery has a tortuous course. The long main path and multiple tortuosity of the vessel are a kind of damper that allows you to maintain the optimal temperature regime of the gonad. It is currently unknown how artificial changes in blood flow affect the functional significance of the gonad.
In recent years, works have appeared that describe endoscopic methods of orchiopexy. The operation is performed laparoscopically in children with the abdominal form of cryptorchidism.
The most commonly used endoscopic orchiopexy method is Fowler-Stephens. It is performed when the testicle is located high in the abdominal cavity and the contralateral testicle is absent or inferior. The operation is performed in two stages. The anatomical prerequisite for the success of Fowler-Stephens orchipexy is a long loop of the vas deferens and a short vascular bundle.
After determining the location of the testicle and its condition during laparoscopy, hemostatic clips are installed, ligating the internal seminal vessels with them at a distance. This completes the first stage of the operation. JA Pascuale et al. (1989) in an experiment found that when the seminal vessels are ligated, blood flow to the testicle decreases by 80% in the first hour, but returns to normal by the 30th day.
6 months after laparoscopic vessel clipping, the patient undergoes the second stage of orchiopexy. The spermatic vessels are ligated and separated from the clips proximally. Then a wide cuff is isolated from the peritoneum of the testicle and the vas deferens, and this complex, after mobilization, is brought down into the scrotum.
An important aspect is the wide selection of the paratesticular layer of the peritoneum. Firstly, this technique allows you to eliminate torsion of the gonad in the process of bringing it down into the scrotum; secondly, there remains the possibility of blood supply to the gonad on the only artery of the vas deferens.
In case of atrophy of the testicle located in the abdominal cavity, laparoscopic orchiectomy is performed.
Prevention of the birth of children with cryptorchidism remains focused on the exclusion of disruptors from the diet of pregnant women and the development of strict indications for the use of hormonal therapy during pregnancy.
Clinical guidelines (European Association of Urology)
- Children with an increased cremasteric reflex (retractile testicles) do not require medical or surgical treatment, but they require regular monitoring until puberty;
- the optimal timing of treatment if cryptorchidism has developed is from 6 to 18 months of the child’s life;
- for non-palpable testicles, laparoscopy is the “gold standard”, since it has almost 100% sensitivity and specificity for identifying intra-abdominal testicles, allowing simultaneous surgical correction;
- adjuvant and non-adjuvant hormonal therapy is not standard treatment. Its use must be individualized for each patient;
- in the presence of an intra-abdominal testicle on one side and a normal contralateral testicle in children over 10 years of age, it is advisable to remove the intra-abdominal testicle due to the high risk of malignancy.
- Federal clinical guidelines “Cryptorchidism”
- Pediatric urology-andrology: Textbook. allowance. — Razin M.P., Galkin V.N., Sukhikh N.K.
- Algorithm for the treatment of children with cryptorchidism Al-Mashat N.A., Kovarsky S.L., Petrukhina Yu.V.
- Surgical treatment of cryptorchidism in children Zholumbaev A.O., Raselbaev K.U.
Lifestyle and recommendations
Even after surgery, it is important to regularly carry out diagnostics - this can be done at home. This way you will be sure that the testicles are developing correctly. Check their position during hygiene procedures - changing diapers and baths. When the boy reaches an older age, be sure to talk to him about his testicles. Explain how he can check them himself. Self-diagnosis is an important skill that can help detect a possible tumor.
Due to the absence of one/both testicles, the boy may worry. These concerns are justified, since the appearance of his genitals differs from the genitals of friends and peers in general. He can see such differences, for example, while changing clothes in a sports locker room. The following tips will help him in this situation:
- the boy must understand the meaning of words such as testicles and scrotum;
- convey information to the child in simple words, explain to him that no matter what, he is still a healthy boy;
- talk to your son about possible testicular replacement;
- teach him the right words if someone around the child teases him or asks about his condition;
- buy loose underwear, it will help hide the condition of the scrotum and make it less noticeable when changing clothes or playing sports.