Triplex scanning of the inferior vena cava and its visceral branches in the ultrasound department of the Yauza Clinical Hospital will help establish an accurate diagnosis, choose the optimal treatment and monitor its results.
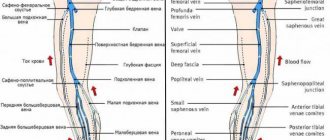
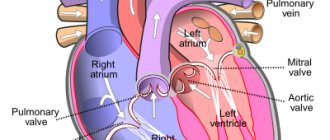
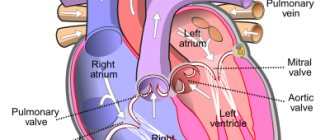
The inferior vena cava is a short, wide vessel into which veins flow, collecting venous blood from the kidneys, liver, and spleen. These are its visceral branches. In addition, it collects blood from the lower extremities through 2 iliac veins. It flows into the right atrium.
At the Yauza Clinical Hospital, the inferior vena cava and its visceral branches are examined using triplex scanning.
The examination is performed by doctors from the Ultrasound Diagnostics Department who have the highest medical category and academic degrees. The department uses modern diagnostic equipment, including Accuvix A30 ultrasound scanners manufactured by Samsung Medison, South Korea and HIVision Prerius - Hitachi, Japan.
Triplex scanning of the inferior vena cava and its visceral branches is a comprehensive ultrasound examination that includes 3 diagnostic techniques.
- ultrasound diagnostics in grey-scale scanning mode (B-mode)
- color Doppler mapping (CDC)
- spectral analysis of blood flow - Doppler ultrasound (USDG)
Method capabilities
Comprehensive ultrasound of the inferior vena cava and its branches allows you to obtain in real time the following information relevant for diagnosis:
- Geometry of the course and location of venous vessels.
- Their permeability.
- Presence of blood clots, narrowing, blockage of veins (occlusions)
- Allows you to identify congenital anomalies in the location and shape of venous vessels.
- Determine the state of regional hemodynamics, its sufficiency and effectiveness.
The purpose of triplex scanning of the inferior vena cava and its visceral branches is to diagnose diseases of the abdominal organs, heart and determine indications for surgical treatment. This study is of particular importance for monitoring installed vena cava filters.
Anatomy of the vena cava
The upper one is located in the chest cavity, namely in its upper part. It is formed by the fusion of two veins - the brachiocephalic veins (right and left). It originates at the level of the first rib to the right of the sternum, goes down, flows at the level of the third right rib into the right atrium. It is adjacent to the right lung, and the aorta passes to the left of it. Behind the superior hollow is the root of the right lung, at the level of the second right rib it is covered by the pericardium. Before its entrance into the pericardial cavity, two veins flow into it: the azygos and the accessory hemi-unpaired.
The inferior vena cava begins in the abdominal cavity. It is formed at the confluence of the iliac veins, goes up, deviates to the right from the aorta towards the diaphragm. It is located in the retroperitoneal space behind the internal organs. Through an opening in the diaphragm it is directed into the chest cavity, from there it goes to the pericardium and flows, like the superior hollow, into the right atrium. The following veins drain into the IVC:
- hepatic;
- diaphragmatic lower;
- adrenal gland right;
- renal;
- right ovarian or testicular;
- lumbar
The inferior vena cava is usually divided into three sections: infrarenal, renal and hepatic.
Preparing for the study
The study is carried out strictly on an empty stomach. Before the examination, you must refrain from smoking and chewing gum. Avoid physical activity on the day of the test.
Patients who are overweight and prone to flatulence should avoid foods that cause gas in the intestines for several days. You should take sorbents, enzymes and other drugs that reduce flatulence for 2-3 days.
If you have indications for examination of the inferior vena cava and its visceral branches, specialists from the Yauza Clinical Hospital will quickly and effectively conduct a triplex ultrasound scan and give a competent opinion.
You can see prices for services
Partial anomalous drainage of the pulmonary veins: mixed type and scimitar syndrome. Clinical notes
- Gavrilov Roman Yurievich
Summary
Partial anomalous pulmonary venous drainage is characterized by the absence of connection of one or more veins to the left atrium. The incidence is 0.3% of all congenital heart defects. Scimitar Syndrome (congenital venolobar syndrome) is one of the rarest and most difficult to diagnose forms of combination of vascular anomalies. It can be combined with other congenital defects of the heart and bronchopulmonary system. A description of three clinical cases of patients with partial anomalous drainage of the pulmonary veins, differing in clinical course, anatomical features and surgical options, is presented. Typical problems that may arise after surgical treatment of partial anomalous drainage of the pulmonary veins are described: superior vena cava syndrome, prosthetic thrombosis, obstruction of the systemic veins. The entire range of surgical interventions used in the primary correction of partial anomalous drainage of the pulmonary veins and endovascular techniques to eliminate complications were used. Careful monitoring of patients who have undergone such interventions will allow timely detection of pathological symptoms for a more detailed examination, including angiography, multispiral computed tomography or magnetic resonance imaging, and planning surgical correction.
Key words: partial anomalous drainage of the pulmonary veins, scimitar syndrome
Financing
.
The study had no sponsorship. Conflict of interest
.
The author declares no conflict of interest. For citation
: Gavrilov R.Yu. Partial anomalous drainage of the pulmonary veins: mixed type and scimitar syndrome. Clinical notes // Clinical and experimental surgery. Journal named after academician B.V. Petrovsky. 2021. Vol. 9, No. 3. Appendix. pp. 90-99. DOI: https://doi.org/10.33029/2308-1198-2021-9-3suppl-90-99
Partial anomalous pulmonary venous drainage (PAPVD) is characterized by the absence of connection of one or more veins to the left atrium. The incidence of PAD is 0.3% of all congenital heart defects (CHD). In this case, the pulmonary veins can drain into the right atrium, coronary sinus, superior or inferior vena cava. Various combinations of abnormal drainage classify it as a mixed type.
Scimitar Syndrome (congenital venolobar syndrome) is one of the rarest and most difficult to diagnose forms of combination of vascular anomalies and occurs in 1-3 cases per 100 thousand newborns [1].
The permanent components of the scimitar syndrome [2] include hypoplasia (dysplasia) of the right lung and partial or complete anomalous pulmonary venous drainage (APPV) of the right lung into the inferior vena cava above or below the diaphragm. At the same time, on a chest x-ray in a direct projection, you can see a curved shadow running parallel to the right contour of the heart, which in appearance resembles a Turkish saber, or scimitar. Non-permanent components include hypo- or aplasia of the pulmonary artery, systemic arterial blood supply to the lungs and pulmonary sequestration.
The first mention of scimitar syndrome, based on autopsy materials, was found in 1836 by G. Cooper and R. Chassinat. In 1912
E. Park described a complex of interconnected anomalies, consisting of hypoplasia of the right lung, pulmonary artery, ADVP and cardiac displacement simulating dextrocardia [3]. In 1956, C. Dotter et al. for the first time made a lifetime diagnosis using angiography [4]. In the same year, N. Halasz et al. described abnormal venous drainage from the right lung and compared it to the Turkish scimitar, but the term “scimitar syndrome” was proposed by C. Neill et al. in 1960 [5]. In 1962, H. Spencer described the combination of “scimitar” syndrome with horseshoe lung, when the basal sections of the right and left lungs are connected by an isthmus of parenchyma behind the heart [6, 7]. In 1973, B. Felson called this anomaly congenital venolobar syndrome.
In cases where the descending right pulmonary vein passes an abnormal route in the right lung, but flows normally into the left atrium, they speak of false scimitar syndrome. With scimitar syndrome, there is varying degrees of hypo- and dysplasia of the right lung, and the pattern of bronchial branching resembles right atrial isomerism.
There is an accessory tracheal bronchus, which is one of the rare malformations of the bronchopulmonary system. It was first described by N. Chiari in 1889. It arises from the lateral outgrowths of the trachea, which usually undergo reverse development during ontogenesis, and more often departs from the right wall of the trachea. The frequency of the anomaly, according to various authors, is 0.5-2% of observations [8]. The accessory tracheal bronchus can:
1) end with a blind diverticulum-like protrusion;
2) ventilate part of the upper lobe;
3) ventilate the additional (supernumerary) lobe of the lung;
4) ventilate the additional third lung;
5) communicate with the cystic degenerative area.
There is hypo- or aplasia of the right pulmonary artery, as well as its local stenoses. Possible narrowing of the anomalous vein at the site of its confluence with the inferior vena cava and sequestration of the pulmonary parenchyma as a result of abnormal systemic arterial blood supply to part of the right lung (usually the lower lobe) from the abdominal aorta. Dual blood supply to the lung combined with pulmonary venous obstruction may more rapidly lead to congestive heart failure and pulmonary arterial hypertension.
Scimitar syndrome can be combined with other congenital heart defects, including septal defects, patent ductus arteriosus, obstructive left heart defects, tetralogy of Fallot, single ventricle of the heart, Bland-White-Garland syndrome, double origin of the great vessels from the right ventricle, etc. [9, 10].
Scimitar syndrome can manifest itself in the neonatal period with severe symptoms or go undiagnosed for a long time against the background of a mild course of the disease [11, 12]. The frequency of respiratory infections depends, among other things, on the degree of lung hypoplasia. Prenatal diagnosis can be made on the basis of displacement of the heart to the right, hypoplasia of the lung and right pulmonary artery in the absence of a diaphragmatic hernia.
Using echocardiography, it is possible to determine the presence of an atrial septal defect (ASD), the direction of pulmonary venous blood flow, and a decrease in the ratio of the diameters of the pulmonary arteries [13].
A typical radiological sign of scimitar syndrome is detected in no more than 3 patients [14]. To assess all components of the syndrome and determine further tactics, cardiac catheterization with angiography, multislice computed tomography (MSCT) or magnetic resonance imaging (MRI) is used. The ability to simultaneously fill the entire venous return system with a contrast agent, as well as an accurate three-dimensional assessment of the anatomy, is very valuable.
With scimitar syndrome, the following types of surgical interventions can be performed: 1) ligation and intersection of the anomalous vein and its anastomosis with the left atrium; 2) transferring the abnormal drainage to the cardiac form and then directing the pulmonary venous return to the left atrium through the IVD defect using a pericardial patch; 3) creation of an intraatrial tunnel between the mouth of the anomalous vein and the septal defect with expansion, if necessary, of the mouth of the inferior vena cava using a patch. In the long-term postoperative period, obstruction of the long intraatrial tunnel is possible due to its complex path and rotation at an angle approaching 180°. When the anomalous vein is located behind the root of the lung, connecting it to the atrium is technically impossible. In this case, it is possible to remove the hypoplastic right lung or transplant it. Compensatory growth of the left lung compensates for the absence of the right. The results of surgical treatment depend on the patient’s age, the anatomy of the defect, associated anomalies and the type of surgical intervention and are not always satisfactory due to the appearance of signs of pulmonary venous return obstruction.
The variability of the clinical course of the described pathology, the features of surgical treatment and further observation allow us to share our own observations.
Clinical case 1. Patient N.,
3 months, during examination at the State Budgetary Institution “VODKB” the diagnosis was made: “CHD. Aneurysm of the membranous part of the interventricular septum. Muscular defect of the interventricular septum. Preserved foramen ovale." The child was observed in the clinic at his place of residence. At the age of 6.5 months, he was examined in the clinic of the State Budgetary Healthcare Institution “Vokkts”, the diagnosis was made: “CHD. Aneurysm of the membranous part of the interventricular septum with two restrictive defects. Preserved foramen ovale."
By the age of 1 year 5 months, the ventricular septal defects closed spontaneously, the aneurysm size was 16x18 mm, without significant impairment of the left ventricular ejection fraction. The heart sizes were normal.
According to MSCT of the chest with contrast, an aneurysm of the basal parts of the interventricular septum measuring 19x23x23 mm was revealed, as well as partial anomalous drainage of the right pulmonary veins: the upper lobe right pulmonary vein with a diameter of 9 mm flows into the superior vena cava, from the middle and lower lobes of the right lung the veins are collected into a single collector with a diameter of 9 mm and flows into the inferior vena cava above the diaphragm. At the mouth, the collector has a diameter of 6 mm. Two small-diameter pulmonary veins from the S6 and S10 segments of the right lung flow into the left atrium. In addition, the right posterolateral hemivertebra T7 was discovered (aplasia of the left half of the body and arch with the absence of the ventral nucleus), fusion of the bodies of the VI and VII ribs with each other, and a bone bridge between their articular heads.
Considering the absence of clinical manifestations of the disease, the satisfactory physical development of the child and the absence of hemodynamic disorders, it was decided to refrain from surgical intervention.
At the age of 2 years 11 months, he was hospitalized at the State Budgetary Healthcare Institution “Vokkts” due to the appearance of signs of stage I circulatory failure (according to N.A. Belokon).
Echocardiography revealed dilatation of the right heart. Aneurysm of the basal parts of the interventricular septum measuring 30×14 mm. The dimensions of the left atrium were at the upper limit of normal values. The root and ascending aorta are not dilated. The pulmonary trunk is not expanded. The aortic valve is tricuspid, unchanged. There is no reverse current. The cusps of the mitral and tricuspid valves are not changed. The papillary muscles of the mitral valve are brought together and dystopic. Mitral regurgitation degree I. Tricuspid regurgitation of the first degree. Estimated pulmonary artery systolic pressure 33 mmHg. Qp/Qs
=1,9.
Based on clinical data and the results of instrumental studies, the diagnosis was made: “CHD. Partial anomalous drainage of the pulmonary veins, mixed type. Aneurysm of the basal and partially middle segments of the interventricular septum. Circulatory failure (CI) stage I" and indications for surgical treatment were determined.
During the operation, standard methods of intraoperative monitoring were used. After pericardiotomy, an inspection of the heart and right pleural cavity was performed, during which it was determined that the right upper lobe pulmonary vein flows into the superior vena cava, the right pulmonary veins from the middle and lower lobes flow into the inferior vena cava at the level of the diaphragm. The artificial blood circulation machine was connected according to the “aorta - vena cava” scheme. Hypothermic cardiopulmonary bypass was started with cooling to 30°. Myocardial protection was performed using intermittent antegrade blood cold potassium cardioplegia. Access was made through the right atrium with an incision from the base of the appendage towards the inferior vena cava. During inspection of the cavity of the right atrium, an open foramen ovale measuring 2x3 mm was discovered. When revising the interventricular septum, its aneurysmal bulge in the basal section with partial adhesion to the septal cusp of the tricuspid valve is noted. The aneurysm was plicated with three sutures on gaskets made from autopericardium. A transseptal approach to the left atrium was performed. During the audit, 2 ostia of the left pulmonary veins were identified. The venous collector with a diameter of 11 mm, above the right dome of the diaphragm, extends to the hilum of the right lung. The anomalous vein is sutured, divided and then anastomosed to the left atrium. Anomalous drainage into the superior vena cava was eliminated using the classical method of H. Warden [14]. The operation was completed as usual.
The postoperative period was complicated by compensated heart failure, which required the administration of dopamine at a dose of 4 mcg/kg per minute, heart rhythm disturbances: transient third degree atrioventricular block on the first day after surgery with transition to second degree atrioventricular block, Mobitz 1, with Wenckebach periodicity, then into first degree atrioventricular block. Sinus rhythm was restored on the 6th day after surgery. The child was extubated 3 hours after surgery and the next day was transferred to the pediatric cardiac surgery department.
According to the results of echocardiography, on the 7th day after surgery, slight dilatation of the right atrium remained. Global contractility of the left ventricle is not impaired. Mitral regurgitation degree I. Tricuspid regurgitation degree II. Estimated pulmonary artery systolic pressure 35 mmHg.
On control MSCT angiography performed 5 months after cardiac surgery, it is determined that 3 veins go into the left atrium from the right lung: 2 with a diameter of 8 mm and one with a diameter of 3.5 mm (Fig. 1).
Rice. 1.
MSCT angiography, VRT reconstruction. An abnormal venous collector of the right lung, draining in the inferior vena cava and having a narrowing at the mouth (yellow arrows below): A - upper lobe pulmonary veins flowing into the superior vena cava (yellow arrows above); B — MSCT of the chest organs. Frontal view. Skeletal abnormalities; B - echocardiography. 4 camera projection. Aneurysm of the basal parts of the interventricular septum; D — MSCT angiography. Axial slice. Pulmonary vein collector anastomosed to the left atrium (yellow arrow)
Fig. 1.
MSCT angiography, VRT reconstruction. Abnormal venous collector of the right lung, draining into the IVC and having a narrowing at the orifice (yellow arrows below): A - upper lobe pulmonary veins flowing into the SVC (yellow arrows above); B - MSCT of the chest. Frontal view. Bone skeletal anomalies; C - echocardiography. 4 cameras projection. Aneurysm of the basal IVS; D - MSCT angiography. Axial slice. The pulmonary vein collector anastomosed to the left atrium (yellow arrow)
According to the results of echocardiography 3 years after the operation, the heart chambers were not dilated. Global contractility of the left ventricle is not impaired. Mitral and tricuspid regurgitation, valvular. There is no acceleration of blood flow at the mouths of the pulmonary veins. The speed of blood flow at the mouth of the superior vena cava is 0.75 m/s.
According to the ECG results 3 years after the operation, no rhythm disturbances were detected. There is an incomplete blockade of the right bundle branch.
Clinical case 2
.
Patient S.
was sent to the clinic of the State Budgetary Healthcare Institution “Vokkts” at the age of 3 years 3 months. The diagnosis was made: “CHD. Secondary MP defect. Partial anomalous drainage of the pulmonary veins into the superior vena cava. Preservation of the left superior vena cava, which flows into the coronary sinus. NK0".
Considering the absence of clinical manifestations of the disease, the satisfactory physical development of the child and the absence of hemodynamic disorders, it was decided to refrain from surgical intervention.
Subsequently, the parents did not follow the recommendations, and the child was not registered with a pediatric cardiologist.
At the age of 11 years and 2 months, she was re-examined in the clinic of the State Budgetary Healthcare Institution “VOCCC”. The reason for the visit was complaints of increased fatigue, shortness of breath during physical activity, and frequent respiratory infections.
According to the ECG results, an atrial rhythm with a frequency of 110 beats/min was recorded.
The results of echocardiography revealed dilatation of the right atrium and ventricle. In the projection of the right atrium cavity there were no pathological currents through the interatrial septum. There was a slight dilatation of the pulmonary artery trunk with a blood flow velocity of 2.0 m/s. The acceleration of blood flow in the left pulmonary artery is 2.7 m/s, in the right pulmonary artery - 2.5 m/s.
According to MSCT of the chest with contrast, partial anomalous drainage of the right pulmonary veins was revealed: the upper lobe right pulmonary vein with a diameter of 7 mm flows into the superior vena cava, from the middle and lower lobes of the right lung the veins collect into a single collector with a diameter of 16 mm and flow into the inferior vena cava vein above the diaphragm. The right superior vena cava has a diameter of 18 mm at the mouth, the left superior vena cava (LSVC) with a diameter of 10 mm flows into the dilated coronary sinus. 2 pulmonary veins flow into the left atrium from the left. The diameter of the mouth of the left superior pulmonary vein is 12.5 mm, the left inferior pulmonary vein is 14 mm. On the right, the pulmonary veins do not drain. The right lung is hypoplastic, there is an accessory tracheal bronchus that ventilates part of the upper lobe (Fig. 2).
Rice. 2.
MSCT angiography: A - frontal section. The pulmonary vein collector draining into the inferior vena cava (yellow arrows); B - frontal section. Structure of the bronchial tree. Left main bronchus (blue arrow). Right main bronchus (red arrow). Accessory tracheal bronchus (yellow arrow)
Fig. 2
. MSCT angiography: A - frontal cut. The pulmonary vein collector that flows into the IVC (yellow arrows); B - frontal cut. The structure of the bronchial tree. Left main bronchus (blue arrow). Right main bronchus (red arrow). Accessory tracheal bronchus (yellow arrow)
Based on clinical data and the results of instrumental studies, the diagnosis was made: “CHD. Partial anomalous drainage of the pulmonary veins. Preservation of the left superior vena cava, which flows into the coronary sinus. Chronic heart failure I functional class II according to NYHA” and the indications for surgical treatment were determined.
Due to frequent acute respiratory infections and violation of recommendations, surgical intervention was postponed several times and was performed at the age of 13 years 1 month.
During the operation, standard methods of intraoperative monitoring were used. After pericardiotomy, an inspection of the heart and right pleural cavity was performed, during which it was determined that the heart was strongly displaced to the right side and rotated, the right upper lobe pulmonary vein with a diameter of 8 mm flows into the superior vena cava 35 mm above the mouth, the right pulmonary veins from the middle and the lower lobes flow into the inferior vena cava at the level of the diaphragm (Fig. 3). The diameter of the left superior vena cava is 10 mm. The left brachiocephalic vein is absent. The artificial blood circulation machine was connected according to the “aorta - vena cava” scheme. Hypothermic cardiopulmonary bypass was started with cooling to 30°. A venous collector with a diameter of 16 mm above the right dome of the diaphragm is isolated to the hilum of the right lung.
Rice. 3.
View of the heart during surgery. The collector of the pulmonary veins, which flows into the inferior vena cava, is placed on a holder
Fig. 3.
View of the heart during surgery. The collector of the pulmonary veins, which flows into the IVC, was taken on the holder
The remoteness of the left atrium did not allow direct anastomosis with the collector. A decision was made to anastomose the venous collector with the right atrium. Myocardial protection was performed using intermittent antegrade blood cold potassium cardioplegia. The right atrium is opened with an incision from the base of the appendage towards the inferior vena cava. During inspection of the cavity of the right atrium, the orifice of the coronary sinus was discovered, expanded to 18 mm. There is no MPP defect. The venous collector is sutured, cut off from the inferior vena cava and then anastomosed to the right atrium. Taking into account the peculiarities of the anatomy, it was not possible to create an IV defect of the required diameter. It was decided to use the coronary sinus. The HDL was divided distal to the cannulation site. Its proximal part was opened with a longitudinal incision to the posterior wall of the left atrium. During the audit, 2 ostia of the left pulmonary veins were identified. The wall between the left atrium and the coronary sinus is excised. Plastic surgery of the posterior wall of the left atrium was performed using a xenopericardial patch. The pulmonary venous return is directed into the left atrium through the coronary sinus opening using a pericardial patch, leaving a 4 mm fenestration. The HDL is lengthened using a thin-walled GORE-TEX prosthesis, 10 mm. Anomalous drainage into the superior vena cava was eliminated using the method of H. Warden [14] with the formation of a T-shaped anastomosis between the right atrial appendage, the right and left vena cava (Fig. 4). The operation was completed as usual.
Rice. 4
. Scheme of the operation: A - general view after atriotomy; B - Warden procedure (yellow arrow). The left superior vena cava is severed (red arrow). The collector is moved abnormally ADLV (blue arrow); B - pulmonary venous return is directed to the left atrium through the opening of the coronary sinus using a patch from the pericardium; D — T-shaped veno-auricular anastomosis (blue arrows)
LSVC - left superior vena cava; RSVV - right superior vena cava; ADPV, abnormally draining pulmonary vein; IVC—inferior vena cava; CS - coronary sinus.
Fig. 4
. Scheme of the operation. A — general view after atriotomy; B - Warden procedure (yellow arrow). The left superior vena cava was cut off (red arrow). The collector of abnormally draining pulmonary veins has been moved (blue arrow); C — Pulmonary venous return is directed to the left atrium through the coronary sinus opening using a pericardial patch; D — T-shaped venoauricular anastomosis (blue arrows)
LSVC - left superior vena cava; RSVC - right superior vena cava; ADPV—abnormally draining pulmonary vein; IVC - inferior vena cava; CS - coronary sinus.
The postoperative period was uneventful. She was extubated 13 hours after surgery and 1 day later transferred to the pediatric cardiac surgery department.
On the 5th day after surgery, an echocardiography was performed. According to its results, slight dilatation of the right atrium remained. Global contractility of the left ventricle is not impaired. Mitral and tricuspid regurgitation of the first degree. The direction of blood shunting through the fenestra is 4 mm from left to right. Laminar blood flows in both atria.
According to the ECG results 10 days after surgery, no rhythm or conduction disturbances were detected.
On control MSCT angiography performed 5 months after cardiac surgery, it is determined that all pulmonary veins drain into the left atrium. There is extensive stenosis in the area of the veno-auricular anastomosis and stenosis of the anastomosis between the left superior vena cava and the prosthesis. VRT reconstruction was performed to evaluate postoperative changes in the anatomy of the pulmonary vessels (Fig. 5).
Rice. 5
. Intraatrial tunnel to the left atrium (green arrow). Right pulmonary veins (blue arrows). Extended stenosis in the area of the venoauricular anastomosis (red arrow). Stenosis of the anastomosis between the left superior vena cava and the graft (yellow arrow)
Fig. 5
. Intra-atrial tunnel to the left atrium (green arrow). Right pulmonary veins (blue arrows). Extended stenosis in the area of the veno-auricular anastomosis (red arrow). Stenosis of the anastomosis between the left superior vena cava and the prosthesis (yellow arrow)
Based on the data obtained, endovascular balloon angioplasty of the superior vena cava was planned, but given the child’s satisfactory health, the parents postponed hospitalization; the operation was performed 2 years 10 months later. The reason for the appeal was complaints of swelling of the upper half of the body and periodic headaches.
On control MSCT angiography performed 3 years 2 months after cardiac surgery, it is determined that all pulmonary veins drain into the left atrium. There is a narrowing of the anastomosis between the superior vena cava and the right atrium appendage up to 4 mm. The proximal segment of the right superior vena cava with a diameter of 18 mm. The left superior vena cava is occluded.
Angiography results revealed subocclusion of the anastomosis between the superior vena cava and the right atrium. Sequential dilatation of the narrowing zone was performed with balloons 8-20 (6 atm), 10-20 (12 atm) and 12-40 (14 atm) with stent implantation 10-19 (15 atm). Marked tissue rigidity was noted at the site of stenosis. According to venography, there is a good fit of the stent with a residual stenosis of 30%. The HDL is occluded, and there is a developed collateral network.
During treatment at this stage, significant clinical improvement was achieved in the form of disappearance of swelling of the upper half of the body and headaches. In the future, endovascular closure of the interatrial communication and repeated dilatation of the stent with a high-pressure balloon are planned to increase the diameter of the venoauricular anastomosis.
Clinical case 3
.
Patient Zh.,
11 years old, during examination at the State Budgetary Healthcare Institution “VODKB” was diagnosed with: “Permanent form of atrial flutter. Invasive electrophysiological study, radiofrequency catheter ablation of the critical zone of the re-entry loop 03/17/2017. Condition after radical correction of congenital heart disease—plasty of the sac defect with relocation of the mouths of the right pulmonary veins to the left atrium 03/11/2015. Complete atrioventricular block. Condition after continuous endocardial stimulation with an Advisa DR MRI pacemaker (VVIR) 01/28/2016.” Until 2021, the girl lived in the Moscow region and was treated at the State Budgetary Healthcare Institution of the Moscow Region Monica named after. M.F. Vladimirsky and the Children's Scientific and Practical Center for Heart Rhythm Disorders OSP NIKIP named after. acad. Yu.E. Veltishcheva. Examined at the clinic of the State Budgetary Healthcare Institution "Vokkts".
Echocardiography revealed dilatation of all heart chambers. Global contractility of the left ventricle is reduced. Aneurysmal dilatation of the basal sections of the right ventricle. The root and ascending aorta are not dilated. The pulmonary trunk is not expanded. The aortic valve is tricuspid, unchanged. There is no reverse current. The cusps of the mitral and tricuspid valves are not changed. There is no mitral regurgitation. Tricuspid regurgitation of the first degree. In the projection of the cavity of the right atrium, pathological currents are not visualized. Estimated pulmonary artery systolic pressure is 37 mm Hg. MSCT angiography is recommended.
According to the results of MSCT of the chest with contrast, a pulmonary arterial pulmonary artery was revealed: all right pulmonary veins are collected into a single collector with a diameter of 15 mm and flow into the inferior vena cava above the diaphragm (Fig. 6). The branches of the middle lobe of the right lung with a diameter of 2 mm flow into the left atrium. The superior vena cava has a diameter of 13 mm at the mouth, the inferior vena cava is expanded to 26 mm. The hepatic veins, 12 mm in diameter, drain into the right atrium. 2 pulmonary veins flow into the left atrium from the left. The diameter of the mouth of the left superior pulmonary vein is 17.5 mm, the left inferior pulmonary vein is 14.5 mm. The right lung is hypoplastic with characteristic changes in the bronchial tree.
Rice. 6.
MSCT angiography, VRT reconstruction. Side view. The collector of the right pulmonary veins (green arrows) drains into the inferior vena cava (blue arrow)
Fig. 6.
MSCT angiography VRT reconstruction. Side view The collector of the right pulmonary veins (green arrows) flows into the inferior vena cava (blue arrow)
Probing results confirmed partial anomalous drainage of the right pulmonary veins into the inferior vena cava—scimitar syndrome. During manometry, the average pressure in the pulmonary artery was 11 mm Hg, in the right ventricle - 9 mm Hg. Blood oxygen saturation in the superior vena cava is 79%, in the inferior vena cava - 92%.
During the operation, standard methods of intraoperative monitoring were used. A peripheral connection of the heart-lung machine was performed according to the “femoral artery - femoral vein” scheme. Resternotomy. There is a total adhesive process in the anterior mediastinum and pericardial cavity. Subtotal cardiolysis was performed. An examination of the heart and right pleural cavity was performed, during which it was determined that the heart was strongly displaced to the right side, and there was pronounced dilatation of the right ventricle. Connecting a heart-lung machine according to the “aorta - vena cava” scheme. Normothermic cardiopulmonary bypass was started. All right pulmonary veins are collected into a single collector and flow into the inferior vena cava at the level of the diaphragm. A venous collector with a diameter of 15 mm above the right dome of the diaphragm is isolated to the hilum of the right lung. The remoteness of the left atrium did not allow direct anastomosis with the collector. Myocardial protection was performed using intermittent antegrade blood cold potassium cardioplegia. The right atrium is opened with an incision from the base of the appendage towards the inferior vena cava. During inspection of the cavity of the right atrium, an independent confluence of the hepatic veins with an orifice widened to 16 mm was discovered; it was additionally cannulated. No MPP defect was detected. There are many prolene sutures on the interatrial septum. Taking into account the anatomical features, a decision was made to create an intraatrial tunnel. An adequate communication has been formed between the atria, the endocardium of the atria is stitched together. During the audit, 2 mouths of the left pulmonary veins and the mouth of the inferior basal vein from the right lung were identified. Pulmonary venous return is directed to the left atrium through the created MRJ defect using an intraatrial tunnel formed from a pericardial patch. The orifice of the inferior vena cava is also dilated using a pericardial patch. The operation was completed as usual.
The postoperative period was uneventful. She was extubated 12 hours after surgery and the next day transferred to the pediatric cardiac surgery department.
According to the results of echocardiography, on the 4th day after surgery, dilatation of the right chambers of the heart persisted. Global contractility of the left ventricle is reduced, at the preoperative level. Mitral and tricuspid regurgitation of the first degree. In the projection of the cavity of the right atrium, pathological currents are not visualized. Laminar blood flow on the tunnel.
On control MSCT angiography performed 14 days after cardiac surgery, it is determined that all pulmonary veins drain into the left atrium (Fig. 7).
Rice. 7.
MSCT angiography, VRT reconstruction. Back view. The right pulmonary veins drain through the intraatrial tunnel into the left atrium (yellow arrows)
Fig. 7.
MSCT angiography VRT reconstruction. Back view. The right pulmonary veins are drained through the intra-atrial tunnel into the left atrium (yellow arrows)
In the first of the presented cases, a diagnosis of scimitar syndrome cannot be made due to the absence of hypoplasia of the right lung, although the pathological anatomy of the right pulmonary veins and surgical tactics correspond to this diagnosis. Even the x-ray picture reflects a typical vascular pattern with a “scimitar”. And yet in the first case we have a mixed type of CHADLV. In the second and third examples, there are constant components of the “scimitar” syndrome - hypoplasia of the right lung and partial or complete ADLV of the right lung into the inferior vena cava above the level of the diaphragm.
When treating our patients, we encountered typical problems that can arise after surgical treatment of PVC: superior vena cava syndrome, thrombosis of the prosthesis, obstruction of the systemic veins, which required repeated operations. The entire range of surgical interventions used in the primary correction of PAD and endovascular techniques to eliminate complications were used. It must be said that when performing similar interventions in patients with simpler types of ADPV, we have never encountered such complications.
Careful monitoring of patients who have undergone such interventions will allow timely detection of pathological symptoms for a more detailed examination, including angiography, MSCT angiography or MRI, and planning surgical correction.
Literature
1. Meza R., Araujo J., Escobar A. et al. Successful surgical repair of scimitar syndrome in a 38-year-old adult // Int. J. Cardiovasc. Thorac. Surg. 2021. Vol. 5, N 6. P. 80-83.
2. Wang H., Kalfa D., Rosenbaum MS, Ginns JN, Lewis MJ, Glickstein JS et al. Scimitar syndrome in children and adults: natural history, outcomes, and risk analysis // Ann. Thorac. Surg. 2018. Vol. 105, N 2. P. 592-598.
3. Park EA Defective development of the right lung, due to anomalous development of the right pulmonary artery and vein: accompanied by dislocation of the heart simulating dextrocardia // Proc. NY Pathol. Soc. 1912. Vol. 12. P. 88-92.
4. Dotter CT, Hardisty NM, Steinberg I. Anomalous right pulmonary vein entering the inferior vena cava: two cases diagnosed during life by angiocardiography and cardiac catheterization // Am. J. Med. 1949. Vol. 218. P. 31-36.
5. Neill C., Ferencz C., Sabiston D. et al. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage. "Scimitar syndrome" // Bull. Johns Hopkins Hosp. 1960. Vol. 107. P. 1-21.
6. Spencer H. Pathology of the Lung. 2nd ed. Oxford: Pergamon Press, 1968. P 73.
7. Figa FH, Yoo SJ, Burrows PE, Turner-Gomez S, Freedom RM Horseshoe lung. A case report with unusual bronchial and pleural anomalies and a proposed new classification // Pediatr. Radiol. 1993. Vol. 23. P. 44-47.
8. Rozenshtraukh L.S., Rybakova M.I., Wiener M.G. X-ray diagnosis of respiratory diseases: a guide for doctors. 2nd ed., revised. and additional Moscow: Medicine, 1987. pp. 118-119.
9. Ruggieri M., Abbate M., Parano E., Distefano A., Guarnera S., Pavone L. Scimitar vein anomaly with multiple cardiac malformations, craniofacial, and central nervous system abnormalities in a brother and sister: familial scimitar anomaly or new syndrome? //Am. J. Med. Genet. 2003. Vol. 116A. P 170-175.
10. Azhari N., Al-Fadley F., Bulbul ZR Tetralogy of Fallot associated with scimitar syndrome // Cardiol Young. 2000. Vol. 10, N 1. P 70-72.
11. Melissa L. Rosado-de-Christenson. Diagnostic Imaging: Chest. 2nd ed. Salt Lake City: Amirsys, 2012. P 174-180.
12. Abbara S. Diagnostic Imaging: Chest. 2nd ed. Salt Lake City: Amirsys, 2013. P. 236-242.
13. Shibuya K., Smallhorn JE, McCrindle BW Echocardiographic clues and accuracy in the diagnosis of scimitar syndrome // J. Am. Soc. Echocardiogr. 1996. Vol. 9. P. 174-181.
14. Gustainyte V., Miller M., Towbin R. et al. Scimitar syndrome // Appl. Radiol. 2021. Vol. 48, N 5. P. 37-39.
15. Warden HE, Gustafson RA, Tarnay TJ, Neal WA An alternative method for repair of partial anomalous pulmonary venous connection to the superior vena cava // Ann. Thorac. Surg. 1984. Vol. 38. P. 601-605.